Abstract
Our knowledge of the molecular mechanisms that regulate hematopoiesis in physiologic and pathologic conditions is limited. Using a molecular approach based on cDNA microarrays, we demonstrated the emergence of an alternative pathway for mature bone marrow cell recovery after the programmed and reversible eradication of CD41+ cells in transgenic mice expressing a conditional toxigene targeted by the platelet αIIb promoter. The expression profile of the newly produced CD41+ cells showed high levels of transcripts encoding Ezh2, TdT, Rag2, and various immunoglobulin (Ig) heavy chains. In this context, we identified and characterized a novel population of Lin-Sca-1hic-Kit- cells, with a lymphoid-like expression pattern, potentially involved in the reconstitution process. Our study revealed novel transcriptional cross talk between myeloid and lymphoid lineages and identified gene expression modifications that occur in vivo under these particular stress conditions, opening important prospects for therapeutic applications.
Introduction
Hematopoiesis is a highly regulated physiologic process in which mature blood cells of all lineages are produced from a single hematopoietic stem cell (HSC). Much is now known about the progressive restriction of cell fate as differentiation proceeds toward particular lineages.1-5 Most studies suggest that once a cell is committed to a lineage, its fate remains unchanged. However, recent work indicates that progenitors can be reprogrammed in vitro to produce other lineages (for a review, see Graf6 ). This process, called intrahematopoietic plasticity, has been clearly demonstrated between B-lymphoid and myelomonocytic lineages.7-12 Interestingly, common lymphoid progenitors (CLPs) have been converted to the megakaryocyte (MK)/erythrocyte lineages by the ectopic expression of the transcription factor GATA-1.13 This transcription factor blocked normal lymphoid differentiation and revealed the plasticity potential of the CLP. However, all evidence of nonlinear differentiation programs has been obtained in vitro, and the nature of in vivo intrahematopoietic lineage cross talk remains unclear.
A transgenic MαIIb-tk mouse model was previously developed in our laboratory to investigate critical events in platelet production.14-17 In these mice, the expression of the thymidine kinase (tk) toxigene is driven by the promoter region of the murine CD41 (GPIIb or αIIb) gene. Ganciclovir (GCV) administration suppresses the entire megakaryocytic lineage, including most stem cells and early progenitors. The reversibility of the system revealed a rapid emergence of CD41+ cells in the bone marrow (BM); however, the molecular basis for this process was not elucidated.
The advent of cDNA microarrays made possible the use of large-scale gene expression screening to define related pathways and networks involved in cellular processes,18-23 particularly in the hematopoietic system.24 In the current study, this technology was used to investigate the transcriptional changes occurring in BM cells of the transgenic MαIIb-tk mice during recovery from ablation of the megakaryocytic lineage. In particular, we report the overexpression of lymphoid-specific genes during the megakaryocytic reconstitution process. These lymphoid transcripts were found mostly in the newly produced CD41+c-kit- cells and in a novel cell population with Lin-Sca-1hic-Kit- phenotype. The results further demonstrated a tight link between the production of CD41+ and lymphoid cells, consistent with previous results showing that lymphoid cells are able to achieve transcriptional reprogramming in response to particular signals.11 These data have major implications in the study of hematopoietic lineage plasticity and might contribute to the effort to improve therapeutic approaches for reconstituting BM after cytotoxic chemotherapy.
Materials and methods
In vivo induction of BM cell knockout
The nucleoside analog GCV (Cymevan; Roche, Nutley, NJ) was administered to MαIIb-tk mice daily by intraperitoneal injection at 0.1 mg/day per gram body weight, as previously described.15,16 Treatment was maintained until the blood platelet count decreased to less than 50 000/mm3. Peripheral blood was collected and counted with an automated hematology analyzer (ABX cell counter; HORIBAABX, SCIL, Montpellier, France). For treatment with the myelosuppressive agent 5-fluorouracil (5-FU), C57/BL6 mice received a single intraperitoneal injection in a volume of 0.3 mL/day (150 mg/kg body weight).
Cell staining and sorting
Hematopoietic subpopulations were defined according to the expression of cell-surface markers and were analyzed and isolated with a MoFlo cell sorter (DakoCytomation, Glostrup, Denmark). Mature red blood cells were lysed before staining. BM cells were stained with unconjugated rat monoclonal antibodies (mAbs) specific for the following lineage (Lin) markers: CD3ϵ (145-2C11), B220 (RA3-6B2), CD11b (M1/70), Gr-1 (RB6-8C5), and TER-119. Lin+ cells were stained with a secondary phycoerythrin (PE)-conjugated anti-rat immunoglobulin (Ig). Cells were then stained with fluorescein isothiocyanate (FITC)-conjugated anti-Sca-1 (D7) and allophycocyanin (APC)-conjugated anti-c-Kit (2B8) mAbs. The HSC-enriched population was phenotypically identified as Lin-Sca-1+c-Kit+. To analyze and isolate the different B-lymphoid lineage populations, a biotinylated anti-Sca-1 mAb, visualized with streptavidin-conjugated PE, FITC, or APC, was used in combination with a PE-conjugated anti-interleukin-7 receptor α (IL-7Rα) (SB/14), FITC-conjugated anti-B220. The following subpopulations were sorted: Lin-Sca-1loc-KitloIL-7R+ for the population enriched in CLP; Sca-1loIL-7R+/loB220lo for Pro-B cells, and Sca-1-IL-7R-B220hi for B lymphocytes. To isolate the megakaryocytic precursors, the APC-conjugated anti-c-Kit mAb was used in combination with an FITC-conjugated anti-CD41 (MWReg30). The following subpopulations were sorted with a minimal purity of 95%: Lin-Sca-1-c-Kit+ fraction enriched for common myeloid progenitors (CMPs); CD41+c-Kit+ and CD41+c-Kit- enriched for committed megakaryocytic cells. The other hematopoietic subpopulations were analyzed with mAbs anti-CD3ϵ, anti-CD4, anti-CD8 (T lymphocytes), anti-CD11b (monocytes), and anti-Gr-1 (granulocytes). For defining hematopoietic cells populations, an mAb directed against the hematopoietic marker CD45 (30-F11) was used. For all experiments, appropriate isotype-matched control mAbs were used to determine the level of background staining. All mAbs were obtained from BD Biosciences (Franklin Lakes, NJ).
Immunofluorescence analysis
FITC-CD41+/APC-c-Kit- cells were sorted and seeded on polylysine slides for 1 hour at 37°C, fixed in 2% paraformaldehyde for 10 minutes, washed with phosphate-buffered saline (PBS), and permeabilized using Triton X-100 (0.1%) for 3 minutes. Cells were washed with PBS and incubated in blocking solution (PBS containing 2% bovine serum albumin) for 1 hour at room temperature before incubation with the rabbit anti-human von Willebrand factor (VWF; Dako Cytomation), biotin-B220 (RA3-6B2), or biotin-CD11b (M1/70) mAbs diluted in blocking solution for 1 hour. The slides were extensively washed and incubated with Cy3-labeled donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) or Cy3-streptavidin (Sigma-Aldrich, Poole, United Kingdom) for 30 minutes in the dark. The slides were washed, and DNA was labeled with 4,6-diamidino-2-phenylindole-2-HCl (DAPI) diluted in the antifading mounting medium Vectashield (Vector Laboratories, Burlingame, CA). Nonspecific fluorescence was assessed by omitting the primary antibodies from the immunolabeling reaction. Cells were examined under a microscope (Axioplan 2 Imaging; Zeiss, Oberkochen, Germany) with a Plan-Apochromat oil-immersion lens (63 × magnification; numerical aperture, 1.4). An AxioCam HRC camera and AxioVision software, version 3.1, were used to capture the images.
Cell culture
Five-week cobblestone area-forming cell (CAFC) assay was performed using murine myeloid long-term culture medium (MyeloCult M5300; StemCell Technologies, Vancouver, BC, Canada) supplemented with 10-6 M hydrocortisone (Sigma). First, the MS-5 layer stromal cell line was plated onto gelatinized 96-well plates at 5 × 103cells/well. Transgenic or wild-type (wt) Lin-Sca-1hi cells were then plated at 100 cells/well onto the feeder layer with 30 replicates. Cultures were maintained for 5 weeks at 33°C and less than 5% CO2 with weekly half-medium replacement. Colony-forming cells (CFCs) were assayed on methylcellulose medium (MyeloCult M3134; StemCell Technologies) supplemented with human thrombopoietin (hTPO), erythropoietin (EPO), interleukin-3 (IL-3), IL-6, and stem cell factor (SCF) in 35-mm dishes. Fixed numbers of transgenic or wt Lin-Sca-1hi cells (1 × 104) were plated and incubated at 37°C under 5% CO2 for 2 weeks. The lymphoid potential of cells was determined by using B-cell culture conditions: 2.5 × 103 Lin-Sca-1hi cells from transgenic or wt mice were plated in triplicate onto an S17 stromal cell layer with Iscove modified Dulbecco medium (IMDM) supplemented with 10% fetal calf serum, penicillin/streptomycin, glutamine, and IL-7 (10 ng/mL). After 1 week, each well was analyzed by flow cytometry to determine the proportion of B-lymphoid cells (using anti-B220 FITC) in the hematopoietic population (using anti-CD45 PE).
DNA microarray hybridization procedure
Total RNA from BM cells was isolated using the RNeasy kit (Qiagen, Hilden, Germany). MoFlo-sorted hematopoietic subpopulations were further amplified using the MessageAmp aRNA kit (Ambion, Austin, TX). The integrity of the RNA samples was verified using an Agilent (Palo Alto, CA) Bioanalyzer. For each hybridization, 20 μg total BM RNA or 2 μg amplified RNA from sorted cell populations were reverse transcribed using the Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) and aminoallyl-dUTP (Sigma). cDNA was labeled by an indirect method using monofunctional NHS-ester Cy3 or Cy5 (Amersham, Buckinghamshire, United Kingdom). Labeled cDNA was then purified by passage through a nucleospin column (Macherey-Nagel, Düren, Germany) and hybridized to the chip manufactured in our CEA microarray platform as described.25
Microarray data analysis
Slides were scanned with a Genepix 4000 microarray scanner (Axon Instruments, Molecular Devices, Sunnyvale, CA). For each hybridized spot, the Cy3 and Cy5 fluorescence values were obtained by using Genepix Pro 4.0 software (Axon Instruments) and were saved as a result file. Spots or areas of the array with obvious blemishes were flagged and excluded from subsequent analysis. Result files were imported into GeneSpring 6.1 software (Silicon Genetics, Agilent) for further analyses. To eliminate dye-related artifacts in 2-color experiments, intensity-dependent Lowess normalization was performed. Each gene expression ratio is reported as an average value, and statistical significance was calculated using the Student t test. Differentially expressed genes were selected as described in “Results.”
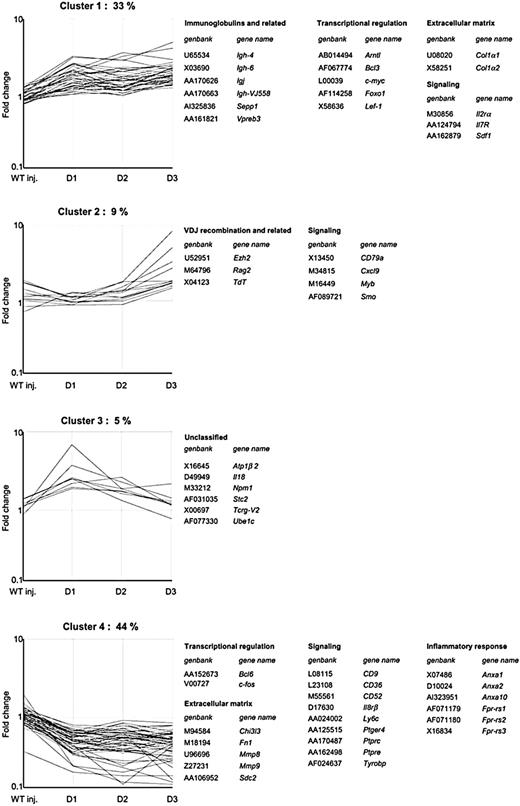
Clusters of genes categorized by expression patterns during BM regeneration. Transcriptional changes were monitored in transgenic mice for 3 days after GCV treatment. Using an ANOVA parametric test (Welch t test) with P < .05, 150 differentially expressed genes were identified. The vertical axis represents the normalized expression ratios. These genes were clustered in 4 groups based on their expression profiles using a QT clustering method and by functional categories using Gene Ontology database. Some representative categories are shown. VDJ indicates Ig heavy chain gene rearrangement. Unclustered gene profiles (9%) were not represented but were included in the complete gene list available in Table S1.
Clusters of genes categorized by expression patterns during BM regeneration. Transcriptional changes were monitored in transgenic mice for 3 days after GCV treatment. Using an ANOVA parametric test (Welch t test) with P < .05, 150 differentially expressed genes were identified. The vertical axis represents the normalized expression ratios. These genes were clustered in 4 groups based on their expression profiles using a QT clustering method and by functional categories using Gene Ontology database. Some representative categories are shown. VDJ indicates Ig heavy chain gene rearrangement. Unclustered gene profiles (9%) were not represented but were included in the complete gene list available in Table S1.
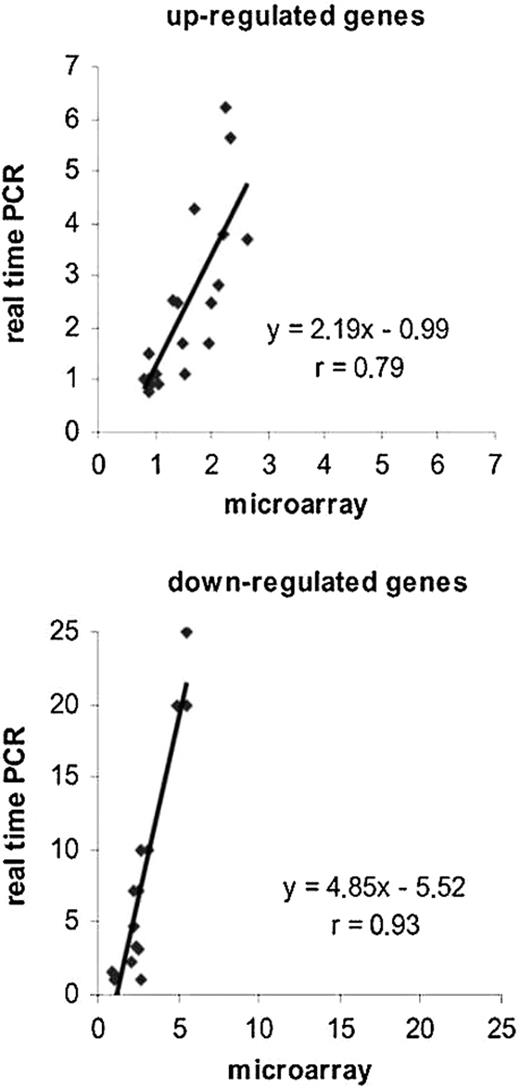
Real-time semiquantitative RT-PCR validation of microarray data. Microarray data were validated by RT-PCR for 5 up-regulated (TdT, Sdf1, Spp-1, Lef1, and Ezh2) and 4 down-regulated (c-fos, Slfn1, CD52, and Mmp9) transcripts. The ratios, expressed as fold change for both methods, were generated by comparing expression levels in BM of treated animals (D1, D2, D3, or wt injected) to the basal levels in those of wt animals. Microarrays ratios (n = 4) were plotted against those calculated with real time RT-PCR (n = 3), and Pearson correlation was determined.
Real-time semiquantitative RT-PCR validation of microarray data. Microarray data were validated by RT-PCR for 5 up-regulated (TdT, Sdf1, Spp-1, Lef1, and Ezh2) and 4 down-regulated (c-fos, Slfn1, CD52, and Mmp9) transcripts. The ratios, expressed as fold change for both methods, were generated by comparing expression levels in BM of treated animals (D1, D2, D3, or wt injected) to the basal levels in those of wt animals. Microarrays ratios (n = 4) were plotted against those calculated with real time RT-PCR (n = 3), and Pearson correlation was determined.
Real-time RT-PCR
RNA samples prepared from total BM and sorted cells were subjected to reverse transcription-polymerase chain reaction (RT-PCR) using SYBR Green Core Reagents (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. The incorporation of the SYBR Green dye into the PCR products was monitored in real time with an ABI PRISM 7700 sequence detection system (Applied Biosystems). Amplification efficiency was determined for each pair of primers by comparison with a standard curve generated with serially diluted cDNA. Target genes were quantified relative to a reference gene using the mathematical model described by Pfaffl.26 All PCR reactions were performed in triplicate. Primer sequences are available on request.
Results
In vivo monitoring of transcriptional changes during BM recovery
Transcriptional events involved in the regeneration of the megakaryocytic cells were monitored in BM cells of MαIIb-tk mice after the GCV-induced eradication of αIIb-expressing cells. To this end, RNA samples prepared from BM cells 24, 48, and 72 hours after cessation of the GCV treatment were converted to cDNA, labeled, and hybridized to the mouse cDNA arrays prepared in house25 in competition with cDNA prepared from wt BM RNA. The use of a common reference for all samples allowed the comparison of the relative expression of each gene. Each hybridization was performed 4 times with a fluorochrome inversion to optimize reliability and to minimize labeling artifacts. The results represent the average of the 4 independent values and were deposited in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) with the GSE1290 accession number.
Criteria applied to select differentially expressed genes were availability of a hybridization value in at least 3 of 4 replicates and analysis of variance (ANOVA) between the transgenic BM samples and the wt samples, with P less than .05. One hundred fifty genes were thereby identified as differentially expressed (Table S1, available on the Blood website, provides the complete list of genes; see the Supplemental Tables link at the top of the online article). The identified sets of genes were grouped into 4 clusters according to their expression profiles during the recovery phase using a QT clustering method (Figure 1). Cluster 1 included genes with continuously increasing expression ratios over the 3-day period. This cluster included the stromal derived factor-1 (Sdf1), which has already been described as involved in megakaryocytic differentiation,27 and several transcripts characteristic of the B-lymphoid lineage, including those coding for various Ig chains, Vpreb3, and IL-7R. Cluster 2 was composed of genes induced mostly on the third day. This cluster also included lymphoid-specific genes such as enzymes involved in the Ig recombination process (Rag2, TdT) and CD79a. Cluster 3 was composed of transcripts that exhibited a rapid increase the first day of the BM recovery, whereas their expression sharply declined over the next 2 days. This category included 2 interleukins: IL-18 and Scy 6. The down-regulated genes were grouped in cluster 4. This set included, among others, c-fos, 2 matrix metalloproteinases (Mmp8, Mmp9), and many genes encoding neutrophil-specific proteins. Probes for genes represented by more than one spot on the chip, such as Ezh2 and TdT, which were present 3 times on the array, yielded comparable induction ratios.
Real-time RT-PCR validation of the microarray results
Real-time RT-PCR was used to verify changes in gene expression obtained by cDNA microarray analysis. Target genes were selected to cover a wide range of modulation ratios and included a few genes previously described in relation to hematopoietic differentiation. The selected up-regulated genes included Sdf1, TdT, Lef1, Spp1, and Ezh2, and the down-regulated genes were c-fos, Slfn1, CD52, and Mmp9. All PCR amplifications were performed in triplicate using specific primers.
To normalize the PCR results 2 internal controls were used, the 18S rRNA and the housekeeping gene Gapdh. We first verified that the 24 Gapdh spots present on each slide yielded fluorescence intensities centered on 1 after normalization and were effectively unchanged for the entire time course of the experiment. Expression levels for the transgenic samples were compared with those of the wt samples at each time point. Results of RT-PCR analysis showed that ratios (n = 3) obtained by this method were higher than those found with microarrays, particularly for the down-regulated genes (Figure 2 and Table 1). Nevertheless, a good correlation was found for up-regulated and down-regulated genes (r = 0.79 and r = 0.93, respectively). Thus, transcriptional changes in gene expression observed with the DNA microarrays were confirmed by real time RT-PCR.
Comparison of microarray and real-time RT-PCR expression ratios
. | WT inj . | . | D1 . | . | D2 . | . | D3 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genes . | Microarray . | PCR . | Microarray . | PCR . | Microarray . | PCR . | Microarray . | PCR . | ||||
| Up-regulated | ||||||||||||
| TdT | 1.0 | 1.1 | 1.5 | 1.1 | 2.0 | 1.7 | 8.6 | 6.1 | ||||
| Sdf1 | 1.1 | 0.9 | 2.0 | 2.5 | 2.1 | 2.8 | 2.6 | 3.7 | ||||
| Spp1 | 0.9 | 0.9 | 2.2 | 3.8 | 1.7 | 4.3 | 2.3 | 6.2 | ||||
| Lef1 | 0.9 | 0.8 | 1.4 | 2.5 | 1.3 | 2.6 | 2.3 | 5.7 | ||||
| Ezh2 | 0.8 | 1.0 | 0.9 | 1.0 | 0.9 | 1.5 | 1.5 | 1.7 | ||||
| Down-regulated | ||||||||||||
| c-fos | 2.7 | 1.0 | 4.9 | 20.0 | 5.6 | 20.0 | 5.6 | 25.0 | ||||
| Sifn1 | 1.0 | 1.4 | 2.5 | 7.1 | 2.7 | 10.0 | 3.1 | 10.0 | ||||
| CD52 | 1.0 | 1.1 | 2.5 | 3.2 | 2.1 | 2.2 | 2.4 | 3.4 | ||||
| Mmp9 | 0.9 | 1.6 | 2.3 | 4.8 | 7.4 | 8.3 | 2.3 | 7.1 | ||||
. | WT inj . | . | D1 . | . | D2 . | . | D3 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genes . | Microarray . | PCR . | Microarray . | PCR . | Microarray . | PCR . | Microarray . | PCR . | ||||
| Up-regulated | ||||||||||||
| TdT | 1.0 | 1.1 | 1.5 | 1.1 | 2.0 | 1.7 | 8.6 | 6.1 | ||||
| Sdf1 | 1.1 | 0.9 | 2.0 | 2.5 | 2.1 | 2.8 | 2.6 | 3.7 | ||||
| Spp1 | 0.9 | 0.9 | 2.2 | 3.8 | 1.7 | 4.3 | 2.3 | 6.2 | ||||
| Lef1 | 0.9 | 0.8 | 1.4 | 2.5 | 1.3 | 2.6 | 2.3 | 5.7 | ||||
| Ezh2 | 0.8 | 1.0 | 0.9 | 1.0 | 0.9 | 1.5 | 1.5 | 1.7 | ||||
| Down-regulated | ||||||||||||
| c-fos | 2.7 | 1.0 | 4.9 | 20.0 | 5.6 | 20.0 | 5.6 | 25.0 | ||||
| Sifn1 | 1.0 | 1.4 | 2.5 | 7.1 | 2.7 | 10.0 | 3.1 | 10.0 | ||||
| CD52 | 1.0 | 1.1 | 2.5 | 3.2 | 2.1 | 2.2 | 2.4 | 3.4 | ||||
| Mmp9 | 0.9 | 1.6 | 2.3 | 4.8 | 7.4 | 8.3 | 2.3 | 7.1 | ||||
The ratios, expressed as fold change for both methods, were generated by comparing expression levels in BM of treated animals (D1, D2, D3, or wt injected) to the basal levels in those of wt animals.
Classification of differentially expressed genes
To give biologic significance to the microarray results, genes identified as modulated during the megakaryocyte recovery phase were assigned to functional categories (Figure 1). Two main categories or genes were represented in total BM during this phase. One group contained genes involved in the extracellular matrix (ECM) remodeling process and included different types of procollagen genes, Mmp8, Mmp9, syndecan 2, fibronectin, and chitinase 3. The second category was composed of transcripts involved in transcriptional regulation. Several of these factors, including Ezh2, Lef1, and Myb, acted in concert in early stages of lymphoid differentiation. The other categories included mostly transcripts characteristic of lymphoid cells, such as those coding for various Ig chains, Vpreb3, CD79a, and IL-7R or enzymes involved in the Ig recombination process (Rag2, TdT), which distinguish lymphoid progenitors from myeloid, erythroid, and megakaryocytic progenitors in the hematopoietic system. Overexpression of these lymphoid genes during the megakaryocytic reconstitution process was thus puzzling. To exclude the possibility of a bias resulting from elimination of the CD41+-expressing cells, a detailed analysis of BM cellularity during the recovery phase was performed.
Increase in the absolute number of Lin-Sca-1hic-Kit- cells and B-lymphoid precursors
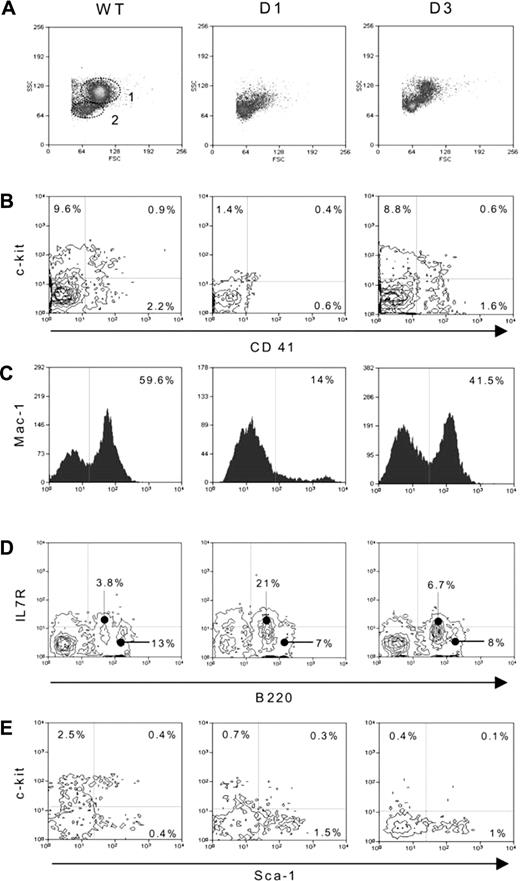
To explain the microarray results, extensive flow cytometry analysis of cells remaining in the BM of GCV-treated mice was performed. The plot obtained from a control mouse showed 2 morphologically distinct groups (Figure 3A), one corresponding to myeloid cells (gate 1) and the other composed mainly of lymphocytes and small lymphoblastlike cells (gate 2). The number of cells in these groups was unchanged for GCV-treated and -untreated wt mice, indicating that GCV had no effect on BM cells (data not shown). In contrast, the depletion of large and granular cells such as MK, granulocytes, and monocytes (gate 1) was evident for transgenic mice the day after the cessation of the GCV treatment. The phenotypic characterization using specific mAbs shown in Figure 3B-C confirmed the decrease of CD41-expressing cells (9 × 105 to 5.7 × 104), c-Kit-expressing cells (3.1 × 106 to 1 × 105), and Mac-1-expressing cells (18 × 106 to 8 × 105). No significant differences between controls and treated transgenic BM were observed for the T-lymphoid lineage using mAbs directed against CD4, CD8, and CD3ϵ (data not shown). Analysis of the B-lymphoid cells on the lymphoblastlike population (gate 2) revealed that the Pro-B-enriched population (Sca-1-/loIL-7Rα+B220lo) increased from 3.8% to 21% (Figure 3D), whereas the percentage of B cells (Sca-1-/loIL-7Rα-B220hi) decreased from 13% to 7%. Analysis of the Sca-1hi-expressing cells confirmed our earlier electron microscopy results showing a significant increase in this population in the GCV-treated transgenic BM.14 We now show that these cells expressed the leukocyte-specific CD45 antigen, excluding the possibility of a mesenchymal origin, but lacked c-Kit or lineage-specific markers (Figure 3E) and that they constituted 90% of the Lin-Sca-1hi cell population after GCV depletion. Interestingly, the increase in this Lin-Sca-1hic-Kit- population contrasted with the decrease in related cell populations, such as the primitive Lin-Sca-++c-Kit+ and Lin-Sca-1-c-Kit+, whose levels remained extremely low during the recovery phase. The latter populations could certainly not have participated in the production of the CD41+ cells, which were already perceptible 3 days after GCV withdrawal (Figure 3B). Moreover, the possibility that flow cytometry analysis was not sufficient to detect small numbers of normal CD41+ progenitor cells was previously assessed by granulocyte, erythrocyte, megakaryocyte, macrophage colony-forming unit (CFU-GEMM), or long-term culture-initiating cell (LTC-IC) cultures, which showed a complete absence of colonies when total transgenic BM was cultured.14-17 Therefore, according to the high number of cells with Lin-Sca-1hic-Kit- phenotype found at the initial stage of the recovery process, this population might represent a critical developmental stage of the reconstitution process.
Morphologic and phenotypical analyses of wt and transgenic BM cells. (A) Cell morphology plots obtained using the forward versus the side scatter signals. Gate 1 corresponds to myeloid cells. Gate 2 contains lymphoid and lymphoblastlike populations. (B) Analysis of megakaryocytic lineage cells by double staining with antibodies against CD41 (FITC) and c-Kit (APC). The figure clearly shows the high decrease of CD41-expressing cells 1 day after GCV withdrawal and their regeneration 3 days after the cessation of treatment. (C) Macromonocytic cells labeled using an anti-Mac-1 (FITC) antibody. (D) Analysis of B-lymphoid lineage on the lymphoblast-like population (gate 2) using antibodies directed against Sca-1, IL-7Rα, and B220. Plots represent the anti-IL-7Rα (APC) and -B220 (FITC) double staining on the Sca-1-/lo (PE) population. (E) Analysis of stem and progenitor cells after lineage-positive cell depletion and double staining with anti-Sca-1 (FITC) and anti-c-Kit (APC) antibodies. (B-E) Numbers in graphs indicate the percentage of cells positive for the given marker.
Morphologic and phenotypical analyses of wt and transgenic BM cells. (A) Cell morphology plots obtained using the forward versus the side scatter signals. Gate 1 corresponds to myeloid cells. Gate 2 contains lymphoid and lymphoblastlike populations. (B) Analysis of megakaryocytic lineage cells by double staining with antibodies against CD41 (FITC) and c-Kit (APC). The figure clearly shows the high decrease of CD41-expressing cells 1 day after GCV withdrawal and their regeneration 3 days after the cessation of treatment. (C) Macromonocytic cells labeled using an anti-Mac-1 (FITC) antibody. (D) Analysis of B-lymphoid lineage on the lymphoblast-like population (gate 2) using antibodies directed against Sca-1, IL-7Rα, and B220. Plots represent the anti-IL-7Rα (APC) and -B220 (FITC) double staining on the Sca-1-/lo (PE) population. (E) Analysis of stem and progenitor cells after lineage-positive cell depletion and double staining with anti-Sca-1 (FITC) and anti-c-Kit (APC) antibodies. (B-E) Numbers in graphs indicate the percentage of cells positive for the given marker.
Cell culture potential of the Lin-Sca-1hi population
To characterize further the Lin-Sca-1hi cells, we evaluated their in vitro clonogenic capacity. We first tested their potential to differentiate into myelomegakaryocytic cells using a CFU assay. A mixture of several hematopoietic growth factors was used to obtain all combinations of mixed myeloid colonies. Results showed that in contrast to the wt Lin-Sca-1hi subpopulation, colonies from the transgenic population were barely detectable, indicating that they had no myeloid potential in vitro. Thus, a separate set of experiments was used to detect the more primitive HSCs. In this assay, CAFCs were observed after 5 weeks of cultures for the wt BM, whereas no colonies were noticed for the transgenic Lin-Sca-1hi cell sample for the same period of time. Again, this is consistent with our previous CFU-GEMM and LTC-IC assays.14-17
We next tested whether the transgenic Lin-Sca-1hi cells might represent a lymphoid progenitor and thus could differentiate into B-lymphocytes. Transgenic Lin-Sca-1hi cells were cultured on confluent monolayers of stromal feeder S17 for 1 week in the presence of exogenously added IL-7. The number and phenotype of the cells produced were evaluated by flow cytometry 7 days later. To exclude contamination of stromal cells, only cells expressing the hematopoietic marker CD45 were considered. Results showed a large reduction in the production of B220+ cells in cultures of transgenic Lin-Sca-1hi cells. Only 3.3% of the cultured transgenic cells counted were B220+ cells, in contrast to 13.7% of the wt population. This indicated that the B-lymphoid differentiation potential of the transgenic Lin-Sca-1hi cells was very low under these conditions and consequently that they could not be considered typical B-cell progenitors. Taken together, the different culture assays revealed that the transgenic Lin-Sca-1hi cells constituted a distinct hematopoietic progenitor.
Hierarchical cluster analysis of gene expression patterns derived from the transgenic Lin-Sca-1hic-Kit- population and the reconstituted CD41+c-Kit- cells. Gene expression patterns of (A) transgenic Lin-Sca-1hic-Kit- (D1 after cessation of GCV treatment) and (B) reconstituted CD41+c-Kit- cells compared with the transcriptional expression profiles of different wt subpopulations, including HSC, myelomegakaryocytic cells (CMP, CD41+c-Kit+, CD41+c-Kit-) and lymphoid B cells (CLP, Pro-B, B lymphocytes). All samples were hybridized independently on cDNA microarrays against total BM of wt animals as a common reference. Hierarchical clustering analysis of the 2474 genes showing significant expression within all the populations was performed with GeneSpring software using a Spearman correlation coefficient (separation ratio, 0.9; minimum distance, 0.001) and was visualized as trees. Tree branch lengths show relative degrees of analogy among cell populations. Each population is represented by a single column, and each gene is represented by a single row. The color designates the expression level compared to that of the reference as represented on the color bar.
Hierarchical cluster analysis of gene expression patterns derived from the transgenic Lin-Sca-1hic-Kit- population and the reconstituted CD41+c-Kit- cells. Gene expression patterns of (A) transgenic Lin-Sca-1hic-Kit- (D1 after cessation of GCV treatment) and (B) reconstituted CD41+c-Kit- cells compared with the transcriptional expression profiles of different wt subpopulations, including HSC, myelomegakaryocytic cells (CMP, CD41+c-Kit+, CD41+c-Kit-) and lymphoid B cells (CLP, Pro-B, B lymphocytes). All samples were hybridized independently on cDNA microarrays against total BM of wt animals as a common reference. Hierarchical clustering analysis of the 2474 genes showing significant expression within all the populations was performed with GeneSpring software using a Spearman correlation coefficient (separation ratio, 0.9; minimum distance, 0.001) and was visualized as trees. Tree branch lengths show relative degrees of analogy among cell populations. Each population is represented by a single column, and each gene is represented by a single row. The color designates the expression level compared to that of the reference as represented on the color bar.
Transcriptional profile of the Lin-Sca-1hi population
To position this novel population into the hematopoietic hierarchy, we compared its expression profile with that of several well-characterized hematopoietic cells by hierarchical clustering. The selected hematopoietic populations included HSCs, the myelomegakaryocytic cell subpopulations (CMP, CD41+c-Kit+, and CD41+c-Kit-), and the B-lymphoid lineage cells (CLP, Pro-B, and B). Messenger RNA prepared from purified cells was reverse transcribed into cDNA, fluorescently labeled, and hybridized against a common reference. We performed 4 independent hybridizations for each population (microarray data are available in the GEO database with the GSE1289 accession number). To perform an unbiased comparison, genes were considered only if a significant hybridization value was obtained for all the studied populations. A set of 2474 genes met this requirement. The expression level of these genes in each hematopoietic cell population was determined and compared by hierarchical clustering. Closely related hematopoietic cells were grouped together, as can be seen in Figure 4A. This type of analysis allowed us to obtain a global image of the transcriptional status of each population of hematopoietic cells and demonstrated that the Lin-Sca-1hic-Kit- population is related to the B-lymphoid lineage subpopulations.
Molecular and cellular characterization of the CD41+c-Kit- sorted cells. (A) Expression levels for 4 megakaryocytic genes (GP3a, Gata1, Fog1, Mpl)inwtand transgenic (Tg) CD41+c-Kit--sorted cells were evaluated by real time RT-PCR. The expression level for each gene was normalized with 18S rRNA, and the fold-change ratio was determined by comparing the expression in reconstituted cells to the basal levels found in wt cells. Ethidium bromide-stained gels are shown. (B) CD41+c-Kit- population was purified from wt transgenic mice at D3 and from 5-FU-injected mice that were allowed to recover for 10 days (5FU). Expression levels for 3 lymphoid genes (TdT, Rag2, Ezh2) were compared by real-time RT-PCR. ▪ indicates Tg; ▦, 5FU. Error bars indicate SD. (C) CD41+c-Kit- was allowed to attach to slides coated with polylysine, fixed, permeabilized, and incubated with antibodies against VWF revealed by Cy3-labeled donkey anti-rabbit IgG, anti-CD11b, and anti-B220 revealed by Cy3-streptavidin. Representative fields were digitized using a fluorescence microscope at 63 × magnification. (D) Expression levels of the 230 transcripts found differentially expressed in transgenic compared with wt CD41+c-Kit- (the complete gene list is available in Table S2). These genes were selected using an ANOVA parametric test (Welch t test; P < .05) with Benjamini and Hochberg multiple testing correction. Two clusters were identified as exhibiting similar expression levels in the transgenic populations. Cluster 1 is composed mainly of genes known to be expressed in lymphoid populations. Cluster 2 shows genes that relate the transgenic populations to wt CLP and pro-B populations.
Molecular and cellular characterization of the CD41+c-Kit- sorted cells. (A) Expression levels for 4 megakaryocytic genes (GP3a, Gata1, Fog1, Mpl)inwtand transgenic (Tg) CD41+c-Kit--sorted cells were evaluated by real time RT-PCR. The expression level for each gene was normalized with 18S rRNA, and the fold-change ratio was determined by comparing the expression in reconstituted cells to the basal levels found in wt cells. Ethidium bromide-stained gels are shown. (B) CD41+c-Kit- population was purified from wt transgenic mice at D3 and from 5-FU-injected mice that were allowed to recover for 10 days (5FU). Expression levels for 3 lymphoid genes (TdT, Rag2, Ezh2) were compared by real-time RT-PCR. ▪ indicates Tg; ▦, 5FU. Error bars indicate SD. (C) CD41+c-Kit- was allowed to attach to slides coated with polylysine, fixed, permeabilized, and incubated with antibodies against VWF revealed by Cy3-labeled donkey anti-rabbit IgG, anti-CD11b, and anti-B220 revealed by Cy3-streptavidin. Representative fields were digitized using a fluorescence microscope at 63 × magnification. (D) Expression levels of the 230 transcripts found differentially expressed in transgenic compared with wt CD41+c-Kit- (the complete gene list is available in Table S2). These genes were selected using an ANOVA parametric test (Welch t test; P < .05) with Benjamini and Hochberg multiple testing correction. Two clusters were identified as exhibiting similar expression levels in the transgenic populations. Cluster 1 is composed mainly of genes known to be expressed in lymphoid populations. Cluster 2 shows genes that relate the transgenic populations to wt CLP and pro-B populations.
Overlapping transcription profiles of CD41+c-Kit- and Lin-Sca-1hic-Kit- transgenic populations
To test whether the Lin-Sca-1hic-Kit- population could be linked to the megakaryocytic lineage recovery in our mouse model, its transcription profile was compared with that of the newly produced CD41+c-Kit- cells isolated from transgenic mice at day 3 (D3). First, the transcription profiles of these cells were compared with those of wt CD41+c-Kit- cells by hierarchical clustering analysis, as described in “Transcriptional profile of the Lin-Sca-1hi population.” Results demonstrated that the transcriptional profiles of these populations clustered together (Figure 4B). Because the analysis of the expression pattern by hierarchical clustering is not sufficient to define the megakaryocytic characteristics, we next tested for the expression of known megakaryocytic genes by semiquantitative PCR analysis in these sorted populations. Results (Figure 5A) showed that the CD41+c-Kit- cells expressed CD61 (GPIIIa or β3), Mpl, Fog-1, and Gata-1 genes and that the expression levels were comparable for wt and transgenic mice, which confirmed their megakaryocytic characteristics. The difference between these 2 populations concerned a group of 230 differentially expressed genes from the 2474 genes studied (ANOVA parametric test, P < .05). This included the transcriptional regulators Spi-1/PU.1 and Ezh2 and several other lymphoid-specific transcripts (Table S2). Moreover, the increase in the level of the lymphoid-specific transcripts encoding Ezh2, TdT, and Rag2 in the CD41+c-Kit- population was assessed by semiquantitative real-time RT-PCR. Results showed that Ezh2, TdT, and Rag2 were, respectively, 7, 5, and 4 times more abundant in the newly formed CD41+c-Kit- transgenic cells than in those of wt cells. Interestingly, these transcripts were not highly increased in the CD41+c-Kit- reconstituted cells after 5-FU treatment (Figure 5B).
The expression of lymphoid genes prompted us to question the nature of the CD41-expressing cells. To this end, the sorted CD41+c-Kit- population was first seeded onto polylysine slides and further analyzed by immunofluorescence. Results indicated that 100% of the sorted population expressed CD41+. We then used an antibody against the VWF specific to maturing CD41+ cells.28 Results are depicted in Figure 5C. Examination of this fluorescence pattern showed that 20% of the CD41+c-Kit- cells were positive for this antibody. The VWF labeling and the large cell sizes were consistent with mature megakaryocytes. Mature megakaryocytes were already observable 3 days after GCV withdrawal. We then assessed whether these CD41+ cells expressed the B220 or the CD11b antigen. The data showed no difference in the proportion of cells in the wt and the transgenic reconstituted CD41+c-Kit- population. Fifty percent of cells were positive for the CD11b marker, and 20% were positive for B220. This composition and the small cell sizes were consistent with multipotent progenitors.
To determine whether this population could have derived from the Lin-Sca-1hic-Kit- population, hierarchical clustering analysis was performed on the basis of the 230 differentially expressed genes. Results, represented in Figure 5D, demonstrated that the transgenic CD41+c-Kit- and the Lin-Sca-1hic-Kit- populations clustered together and shared numerous features. In particular, 2 groups of genes were modulated only in these 2 populations (cluster 1 and 2; Figure 5D). Interestingly, cluster 1 included mostly genes coding for several Ig heavy chains, IL-7R, IL-2Rα, and IL-2Rβ, which are commonly expressed in the lymphoid lineage. Taken together, these results suggested that the Lin-Sca-1hic-Kit- population with lymphoid and progenitor characteristics could represent part of the pathway involved in CD41+c-Kit- regeneration.
Discussion
In this work, daily monitoring of transcriptional variations in MαIIb-tk transgenic mice with cDNA microarrays demonstrated gene expression modifications occurring in BM cells during recovery from toxigene-mediated cell knockout and identified a Lin-Sca-1hic-Kit- population of cells with lymphoid characteristics involved in the in vivo reconstitution process.
The study of BM regeneration after eradication of αIIb-expressing cells assessed in its in vivo environment revealed the involvement of 150 genes in this process; 79 were up-regulated, and 71 were down-regulated (Figure 1). One of the most highly represented categories included genes involved in the ECM remodeling process and in cellular interactions with the BM microenvironment. Notably, transcripts encoding 2 fibrillar procollagens (Col1α1, Col1α2) were induced in parallel to the down-regulation of the mRNA encoding their substrate, the neutrophil collagenase (Mmp8), and other ECM transcripts such as gelatinase B (Mmp9) and fibronectin. The other categories were characterized by the abundance of lymphoid-related genes. This included various Ig chains, Rag2, TdT, Vpreb3, Ezh2, Lef1, IL-7R, and CD79a. Rag2 and TdT are enzymes directly involved in Ig gene recombination.29 Vpreb3 regulates pre-B cell expansion and allelic exclusion at the Ig heavy-chain locus and also mediates the selection of Ig heavy-chain variable gene segments.30 Lef1 is a transcription factor expressed in pro-B and pre-B cells involved in the allelic exclusion process by activating the Rag2 promoter.8,31 Ezh2, an essential regulator of embryonic development, controls B-cell development through chromatin modification and Ig heavy-chain gene rearrangement.32 CD79a is a useful cell-surface marker found almost exclusively on B cells. Its expression precedes Ig heavy-chain gene rearrangement and is lost during the late stage of B-cell differentiation.33 IL-7R activity is both necessary and sufficient for CLPs to differentiate into early B-lineage precursors.34 All these transcripts are part of the signature of pro-B cells and were unexpectedly increased during the megakaryocytic regeneration process.
Understanding of this apparent paradox arose from the study of purified BM populations and transcriptional profiling. In particular, we identified a Lin-Sca-1hic-Kit- population present in high amounts at the initial stage of the megakaryocytic lineage recovery. The clonogenic properties of these cells indicated that they constituted a novel CD45-expressing population. These cells have morphologic features of undifferentiated cells14 ; they are phenotypically similar to those observed after 5-FU treatment35 and to the new class of quiescent HSCs that are in equilibrium with the active Lin-Sca-1+c-Kit+ population.36,37 To characterize them further, we analyzed their transcription profile and compared it with other hematopoietic populations by hierarchical clustering. This technique has been used previously to produce comprehensive representation of cellular status,19,20,38 to compare gene expression patterns of human embryonic stem cells and pluripotent germ cell tumors,39 and to identify commonalties between mouse embryonic and adult stem cells.40,41 In this study, we showed that each cell population was characterized by a clearly distinguishable pattern of gene expression and that hematopoietic cells belonging to the same lineage clustered together. The transcription profile of Lin-Sca-1hic-Kit- cells clustered closely to those of the B-lineage subpopulations and to the newly produced CD41+ cells. In particular, both populations shared many transcripts, suggesting that they might belong to the same differentiation pathway. Interestingly, most of these were typical of B-lymphoid populations. The identification of these transcripts in the CD41+ cells suggested that a subset of B-lymphoid cells expressed this antigen. This was confirmed by immunofluorescence studies showing the coexpression of B220 and CD41 antigens. CD41 was first considered a specific marker of committed megakaryocytic cells.42 However, its expression was found to be less restricted than originally thought. Its promoter was shown to be active in uncommitted cells,15,17 and its expression was found in multilineage progenitor cells.43-45 Neither the differentiation potential of such cells nor the functional roles of CD41 expression on progenitors from the erythroid, myeloid, or lymphoid lineage has yet been explained. The fact that a primitive population of hematopoietic cells is capable of maturation along multiple lineages is widely accepted, and, as we showed in this study, this population exists in physiological conditions. However, major transcriptional differences were found in this population during recovery from toxigene myelosuppression. These concerned mainly the expression of many lymphoid transcripts not found in 5-FU-regenerating CD41+ cells (Figure 5B). Interestingly, recent studies revealed the presence of 2 megakaryocytic progenitors expressing the CD41 antigen that could not be recognized by their morphology as megakaryocytes.46 Moreover, these investigators detected a minor level of B-cell progenitor activity after the mice underwent CMP transplantation.
The comparison of the toxigene and the 5-FU treatments highlight several differences in the myeloid cell recovery pathways. First, analysis of cells remaining in total BM after 5-FU treatment showed that not all the CD41+ cells were eradicated and that the lymphoid and megakaryocytic populations were decreased to similar extents (data not shown), which confirms the nonlineage specificity of the 5-FU treatment. This contrasted with the toxigene-directed cell eradication largely discussed in our previous studies and further demonstrated here by the presence of T- or B-lymphocyte precursors in the BM after toxigene myelosuppression. Second, the transgenic model has a rapid in vivo reconstituting ability. Initial histologic analysis of transgenic BM showed that 3 days after cessation of the treatment, newly synthesized CD41+ cells with MK morphology were present in the BM.14 This was confirmed by immunofluorescence staining and flow cytometric analysis in the present study. In contrast, recovery from 5-FU treatment in which all myeloid progenitors were amplified in the BM took more than 5 days.47 Third, after 5-FU administration, the increase in Lin-Sca-1+c-Kit- cells occurred in parallel to an increase in Lin-Sca-1+c-Kit+ cells, consistent with their use in BM transplantation. It has been demonstrated that the recovery of CD41+ cells after 5-FU treatment originates from a class of Lin-Sca-1+c-Kit+ primitive stem cells or early megakaryocyte precursors that are not killed by the drug.48 This contrasts with the transgenic model in which most of the Lin-Sca-1+c-Kit+ and CD41+ precursors cells were eradicated, and the organism is left to take an alternative route to rapidly recover.
Taken together, our results are consistent with previous data showing that lymphoid and myeloid promiscuity can occur in vitro49 or in hematologic malignancies,50-52 and they support results showing that even though CMP and CLP normally generate mutually exclusive progeny, lineage commitment is not absolute.13 In fact, these immature hematopoietic progenitors coexpress myeloerythroid genes as well as T- and B-lymphocyte-specific genes,49 and their hidden multipotentialities might be revealed under particular stress conditions.
Our genomewide analysis constitutes a first step toward unraveling gene expression cascades that govern myeloid cell production in stress situations. The evidence of a novel transcriptional and functional progenitor cell that could be involved in the regeneration pathway suggests the existence of a novel regulatory mechanism by which lineage-affiliated differentiation programs might be activated at the transcriptional level in an unrelated lineage in vivo. Our study opens new possibilities for understanding hematopoietic system homeostasis and its adaptation to environmental variations, especially under conditions of BM failure.
Prepublished online as Blood First Edition Paper, June 9, 2005; DOI 10.1182/blood-2004-10-3975.
B.J. and T.K. contributed equally to this work.
Supported by funds from the Commissariat à l'Energie Atomique (CEA) and the Association Française contre les Myopathies (AFM) and by a postdoctoral training grant from Genopole-Evry.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs A. Galy and E. Lauret for their assistance in cell culture studies, J.-M. Egly and Y. Senis for critical reading of the manuscript, and Dr G. Marguerie, who initially established the CEA microarray platform. We also thank the staff of the Genethon mouse facility and P. Flament and V. Neuville for outstanding work in animal care at the CEA-FAR mouse facility. All the animal studies reported were performed in accordance with current French regulations (Décret no. 87-848 modifié, Ministère de l'Agriculture).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal