Abstract
During early mouse embryogenesis, fetal liver kinase-1 (Flk-1), a receptor for vascular endothelial growth factor, and Runx1, a runt domain transcription factor, have prerequisite roles in the generation of hematopoietic lineages. Flk-1 expression is maintained in successive stages from mesodermal to endothelial cells and is down-regulated in nascent hematopoietic cells, whereas Runx1 (Runt-related transcription factor 1) is expressed in embryonic sites of hematopoietic cell de novo generation and in practically all hematopoietic organs. Here we show that Runx1 represses Flk-1 during the development of hemogenic endothelial cells into hematopoietic cells. We established embryonic stem cell clones carrying the Venus gene, a modified version of yellow fluorescence protein, in the Runx1 locus and cultured them on OP9 cells. Flk-1+ cells appeared on day 3.5, and Runx1+ cells first appeared from the Flk-1+ fraction on day 4.5. The Flk-1+Runx1+ cells rapidly stopped expressing Flk-1 with further incubation and eventually gave rise to CD45+ or TER119+ cells. Runx1 repressed Flk-1 promoter transcriptional activity in an endothelial cell line, and this repression required intact DNA-binding and transactivating domains of Runx1 protein. The repressor activity of Runx1 endogenous Flk-1 was also confirmed overexpressing Runx1 in embryonic stem cell differentiation cultures. These results provide novel insight into the role Runx1 during the development of hematopoietic cell lineages.
Introduction
During mammalian embryogenesis, 2 waves of hematopoiesis develop in close association with the development of endothelial cells (ECs). The first wave, known as primitive hematopoiesis, occurs transiently in association with the formation of extraembryonic tissue structures called blood islands that consist of blood cells and surrounding angioblasts. The second wave is known as definitive hematopoiesis, during which the hematopoietic stem cells that function throughout life are generated. Accumulating evidence suggests that at least some definitive hematopoietic cells (HPCs) are generated from a special subset of ECs,1,2 designated hemogenic ECs. This notion was first suggested by histologic observations in many species of clusters of blood cells that attached to the luminal wall of the dorsal aorta and that appeared to be budding from the ECs at the onset of definitive hematopoiesis.3-7 More recently, the existence of hemogenic ECs was demonstrated in experiments in which multilineage HPCs, including lymphocytes, were induced from cells that were sorted from embryos according to their expression of EC markers.8-12
Fetal liver kinase-1 (Flk-1), also known as vascular endothelial growth factor (VEGF) receptor-2, is a receptor tyrosine kinase that is indispensable for the differentiation of HPCs and ECs.13,14 Its expression is detected during successive stages from early lateral mesoderm cells to ECs.15,16 Previously, we and others16,17 demonstrated that early Flk-1–expressing cells are the diverging point between HPCs (both the primitive and the definitive) and ECs. Flk-1+ vascular endothelial (VE) cadherin-cells representing lateral mesodermal cells are induced first from embryonic stem (ES) cells, and, as mentioned, the definitive HPC lineage diverges from the Flk-1+ cells after the induction of EC marker expression.18 During mesodermal specification, early Flk-1 expression persists mainly in the EC lineage; other lineages, including hemogenic ECs and HPCs, quickly down-regulate Flk-1, and this down-regulation serves as one of the markers of hematopoietic commitment.16,17
The Runx1 gene encodes the DNA-binding subunit (α-subunit) of a polyomavirus-enhancer binding protein 2 (PEBP2) transcription factor complex and has a critical role in the generation of definitive hematopoeisis.19 At midgestation in the mouse, Runx1 (Runt-related transcription factor 1) is expressed by cell clusters on the ventral wall of dorsal aorta, and cell-sorting experiments have demonstrated that the hematopoietic progenitors were exclusively within the Runx1+ population of the dorsal aorta, suggesting that Runx1 is expressed by hemogenic ECs.20 In addition, Runx1 is thought to have a role in the EC–HPC transition because of observations that the budding cells are absent in Runx1 knockout mice.21 Because Runx1 has a role in the differentiation of HPCs from ECs, it is likely involved, either directly or indirectly, in the process of down-regulation of EC-specific molecules such as Flk-1. Indeed, though endogenous Flk-1 is expressed in hemogenic and nonhemogenic ECs, an Flk-1 regulatory unit, consisting of a 5′ flanking region and a 3′ portion of the first intron of the Flk-1 gene, is suppressed in hemogenic ECs that express Runx1, whereas it is active in fully committed ECs that do not express Runx1.22 These observations prompted us to investigate whether there is cross-talk between Runx1 and Flk-1 during the development of definitive hematopoiesis. Our results suggest that Runx1 serves as a repressor of Flk-1 in mesoderm and ECs.
Materials and methods
Construction of the Runx/Venus knock-in targeting vector and isolation of the knock-in clones
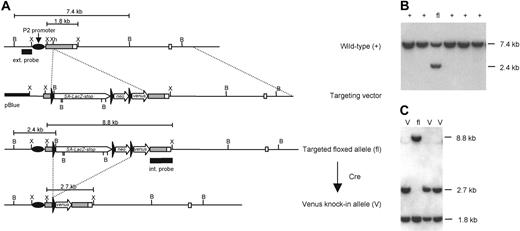
A 12–kilobase (kb) genomic fragment containing the proximal promoter P2 and 2 exons of the Runx1 gene was isolated from a mouse 129/Sv λ genomic library and subcloned into pBluescript (Stratagene, La Jolla, CA). A floxed gene reporter cassette was inserted into the XhoI site in the P2-5′ untranslated region (P2-5′UTR). The insert consists of a 1.7-kb splice acceptor sequence from the mouse Engrailed-2 gene,23 the lacZ reporter gene, transcription termination signals, and a floxed HSV-TKneo gene cassette (pL2-neo vector; a gift from Dr H. Gu, National Institute of Allergy and Infectious Diseases/National Institutes of Health, Rockville, MD). The Venus coding sequence,24 lacking polyA signals, was juxtaposed down-stream of the floxed insert (Figure 1A). The targeting vector was linearized at a unique NotI site before electroporation of ES cells. CCE ES (107) cells were electroporated with 40 μg linearized Runx1 targeting construct, and stably transfected clones were selected with 400 μg/mL G418. Correctly targeted clones were identified by Southern blot analysis of genomic DNA after digestion with BamHI and hybridization with the 5′ external probe (Figure 1B). Single-copy integration of the vector DNA to the clones and the integrity of the targeted Runx1 locus were confirmed by Southern blot analysis with an internal genomic probe spanning Runx1 P2-5′UTR and the adjacent coding region. The floxed insert cassette was removed from correctly targeted ES clones by transient expression of Cre recombinase using pIC-Cre (a gift from Dr H. Gu) (Figure 1C).
Plasmids and transient transfections
The pFlk-1prom/GL3 vector was generated by insertion of the -640 bp/+299 bp (base pairs) promoter fragment of the pGLacZ-Flk-1 promoter/enhancer25 into the KpnI and HindIII restriction sites of the pGL3 vector (Promega, Madison, WI). The pcDEFBOS/GL3 was generated by insertion of the KpnI-XbaI fragment of the pGL3 vector into the KpnI and XbaI restriction sites in the pcDEF3 vector. Deletion mutants of Flk-1 promoter, -474/299, -332/299, -240/299, and -475/-240 were made by cutting out the fragments between KpnI and BalI, KpnI and EcoT14I, KpnI and BstXI, or BalI and BstXI of pFlk-1prom/GL3 vector, respectively, and by self-ligation of the remnant vectors. Point mutations that disrupt the putative Runx1-binding sites at -201, +22, and +39 of the promoter were generated by polymerase chain reaction (PCR)–based site-directed mutagenesis. The mutations at -201 changed the sequence from ACCA to CTTA, those at +22 changed the sequence from ACCC to CTTC, and those at +39 changed the sequence from GTGTT to CTAAG. The introduced mutations and the integrity of nonmutated regions were confirmed by sequencing of cloned PCR products. Expression vectors for Runx1, Runx1 mutants, and PEBP2β26,27 were kind gifts from Dr Motomi Osato (Institute of Molecular and Cell Biology, National University of Singapore).
Generation of VenusYFP (Venus) knock-in ES cells. (A) Targeting strategy used to insert the Venus gene reporter under P2 proximal promoter of Runx1 locus. Exons are presented as boxes; P2-5′UTR is gray, coding regions are light. Black triangles are loxP sites; the 5′ external and internal genomic probes used for screening are indicated, as are the P2 proximal promoter of Runx1. SA-LacZ-stop, mouse Engrailed2 splice acceptor—LacZ—transcription stop cassette; neo, HSV-TK–neoR–polyA cassette; pBlue, pBluescript II SK+; B, BamHI; X, XbaI; Xh, XhoI. (B) Southern blot analysis of the targeted allele. ES genomic DNA was digested by BamHI, blotted, and hybridized with the 5′-external genomic probe. The properly targeted allele generates a 2.4-kb DNA band. (C) Southern hybridization analysis of Runx1fl/+ ES cells subjected to Cre recombinase–dependent excision of the floxed (loxP flanked) cassettes. Internal genomic probe hybridizes with 1.8-kb, 2.4-kb, and 8.8-kb XbaI genomic fragments derived from wild-type (+), Venus (V) knock-in, and floxed (fl) Runx1 alleles, respectively.
Generation of VenusYFP (Venus) knock-in ES cells. (A) Targeting strategy used to insert the Venus gene reporter under P2 proximal promoter of Runx1 locus. Exons are presented as boxes; P2-5′UTR is gray, coding regions are light. Black triangles are loxP sites; the 5′ external and internal genomic probes used for screening are indicated, as are the P2 proximal promoter of Runx1. SA-LacZ-stop, mouse Engrailed2 splice acceptor—LacZ—transcription stop cassette; neo, HSV-TK–neoR–polyA cassette; pBlue, pBluescript II SK+; B, BamHI; X, XbaI; Xh, XhoI. (B) Southern blot analysis of the targeted allele. ES genomic DNA was digested by BamHI, blotted, and hybridized with the 5′-external genomic probe. The properly targeted allele generates a 2.4-kb DNA band. (C) Southern hybridization analysis of Runx1fl/+ ES cells subjected to Cre recombinase–dependent excision of the floxed (loxP flanked) cassettes. Internal genomic probe hybridizes with 1.8-kb, 2.4-kb, and 8.8-kb XbaI genomic fragments derived from wild-type (+), Venus (V) knock-in, and floxed (fl) Runx1 alleles, respectively.
Bovine aortic endothelial cells (BAECs; 2 × 104) were seeded in 12-well plates 24 hours before transfection. Cells were transfected using FuGENE6 (Roche Diagnostics, Mannheim, Germany) with 200 ng reporter plasmid and 200 ng expression vectors. Luciferase activity was normalized for transfection efficiency with the cotransfected 100 ng pRL-null (Promega), as previously described.28 Luciferase assays were performed 48 hours after transfection using the dual luciferase reporter assay system (Promega) according to the manufacturer's protocols. All transactivation experiments were repeated at least 3 times.
ES lines with conditional Runx1 expression
The modified version of the tetracycline (Tet) regulatory system was used to induce Runx1 expression in ES cell clones essentially as described previously.29 E14tg2a ES cells were stably transfected with the tetracycline transactivator (tTA). A tet-regulatable Runx1 construct was generated by inserting the mouse Runx1 cDNA into the EcoRI site of pUHD10-3IRESEGFP29 (a kind gift from Dr Takumi Era, Center for Developmental Biology, Riken, Kobe). Tetracycline-inducible Runx1-expressing cell lines were established by stable transfection of pUHD10-3Runx1IRESGFP into the parental cells, and the ES cell lines with integrated pUHD10-3IRESGFP constructs were used as controls.
Cell culture and in vitro differentiation of ES cells
Culture of ES cells and OP9 stromal cells was performed as previously described.10,22 Induction of ES cell differentiation was also performed as described previously.10,22 Briefly, 3 × 104 undifferentiated ES cells were transferred to each well of a type 4 collagen-coated, 6-well plate (Biocoat; Becton Dickinson Labware, Bedford, MA) and were incubated in α minimum essential medium (Gibco BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum and 50 μM 2-mercaptoethanol in the absence of leukemia inhibitory factor (LIF). Alternatively, cells were cultured on confluent OP9 cell layers in 6-well plates (Becton Dickinson Labware) at a density of 1 × 104 cells per well to induce differentiation. For hematopoietic differentiation, the total ES cell/OP9 culture was split 1:100 after 5 days of differentiation and reseeded on fresh OP9 cell layers in the presence of the following cytokines: mouse granulocyte–colony-stimulating factor (G-CSF) (100 ng/mL), murine stem cell factor (SCF) (100 ng/mL), mouse interleukin-3 (mIL-3) (200 U/mL), human erythropoietin (EPO) (2 U/mL), and human VEGF (10 ng/mL). For B-cell lineage differentiation, the medium for ES cells cultured on OP9 stroma was supplemented with mIL-7 (20 ng/mL) and mFlt-3L (murine Fms-like tyrosine kinase 3 ligand; 20 ng/mL). In both cases, the cytokine-supplemented cultures were maintained for 14 days before fluorescence-activated cell sorter (FACS) analysis. Recombinant IL-3, SCF, and IL-7 were purchased from Pepro Tech (Rocky Hill, NJ); recombinant G-CSF, EPO, VEGF, and Flt-3L were purchased from R&D Systems (Minneapolis, MN). Cultured cells were harvested with cell dissociation buffer (Gibco BRL) and analyzed.
Monoclonal antibodies, cell staining, and sorting
The monoclonal antibody (mAb) AVAS12 (anti–Flk-1) was purified from hybridoma culture supernatants using protein G-Sepharose columns (Pharmacia, Uppsala, Sweden) and was labeled with allophycocyanin (APC) by standard methods.15 Fluorescein isothiocyanate (FITC) anti-CD45 (common leukocyte antigen), FITC anti-Ter119 (erythroid marker), APC–anti–c-Kit, APC–anti–CD45, APC–anti–CD11b (Mac-1, monocyte/macrophage marker), APC–anti–Ly6G/C (Gr-1, granulocyte marker), and APC–anti–CD45R (B220, B-cell marker) were purchased from Pharmingen (San Diego, CA). Nonspecific antibody binding was blocked using normal mouse serum, and cells were labeled with combinations of the mAbs. Stained cells were resuspended in Hanks balanced salt solution (Gibco BRL) containing 1% bovine serum albumin (Sigma-Aldrich Chemical, St Louis, MO) and 5 μg/mL propidium iodide (PI; Sigma-Aldrich) to exclude dead cells. Cells were analyzed and sorted by FACS Vantage or Aria (Becton Dickinson Immunocytometry Systems, San Jose, CA) using the CellQuest software (Becton Dickinson Immunocytometry Systems).
Immunoblotting
Runx1 proteins were detected with rabbit polyclonal serum against Runx1 (Oncogene, Parmastadt, Germany) followed by treatment with a horse-radish peroxidase–conjugated anti–rabbit immunoglobulin G (IgG) secondary antibody.
RT-PCR
Total RNA was prepared from sorted cell populations or cultured cells using Isogen (Nippon Gene, Toyama, Japan). RNA was reverse-transcribed with Superscript II reverse transcriptase (Gibco BRL) and oligo (dT)12-18 primer (Gibco BRL) according to the manufacturer's instructions. PCR assays were performed in the reaction mixture containing 1 × ExTaq Buffer (Takara Shuzo, Osaka, Japan), 200 μM dNTP (Pharmacia), 25 U/mL ExTaq DNA polymerase (Takara Shuzo), several dilutions of cDNA, and 2 μM specific primers. Sequences of primers for Flk-1 were as follows: forward, 5′-GTGGATCTGAAAAGACGC-3′; reverse, 5′-CATTCTTCTCCGATAGG-3′. Runx1 semiquantitative reverse transcription–PCR (RT-PCR) was performed using the Cell-to-cDNA II Kit (Ambion, Austin, TX). Sequences of primers for Runx1 and ribosomal protein 13 large subunit were as follows: Runx1 forward, 5′-CAATCGGCTTGTTGTGATGC-3′; reverse, 5′-TTCATCGTTGCCTGCCATGAC-3′; ribosomal protein 13 large subunit forward, 5′-GCTCCAAGCTCATCCTGTTC-3′; reverse 5′-GGAGACTGGCAAAAGCCTTA-3′. PCR products were electrophoresed through a 1% agarose gel and were stained with ethidium bromide.
Results
Establishment of Runx1-Venus knock-in ES cell lines
First, we investigated the relation between Runx1 and Flk-1 expression during HPC development. Although Flk-1 expression is detectable by a combination of fluorescence-labeled specific mAbs and flow cytometry, monitoring Runx1 expression requires markers that indicate its expression. For this purpose, we introduced the Venus24 gene reporter into one of the Runx1 alleles. The Venus gene was placed in the P2-5′UTR under the control of the Runx1 proximal promoter P2, which is active before the Runx1 distal P1 promoter during ES cell differentiation in vitro30 (Figure 1A). Venus is a modified version of yellow fluorescence protein with an improved efficiency and speed of maturation that substantially increases the sensitivity of gene reporter analysis. The HSV-TKneo-positive selection cassette was deleted to prevent possible interference with the proximal promoter (Figure 1A, C). No polyadenylation signals were inserted downstream of the Venus coding sequence, enabling the knock-in transcripts to use functionally important Runx1 3′-UTR31 and to use an endogenous internal ribosome entry sequence (IRES) in the P2-5′UTR.32 To minimize the interference with IRES and proximal promoter functions, we introduced the Venus gene into the UTR approximately 1200 bp upstream from the translation initiation codon and approximately 400 bp downstream from the proximal promoter transcription start. This makes the recombinant transcripts effectively bicistronic and thereby reduces possible Runx1 gene dosage effects. The kinetics of early differentiation of the recombinant Runx1Venus/+ ES clones in terms of the changes in expression of Flk-1 were identical to the kinetics observed in the experiments using wild-type ES cells (data not shown), whereas the diagnostic feature of Runx1 haploinsufficiency is significantly earlier emergence of Flk-1 mesoderm marker during ES cell differentiation in vitro.33
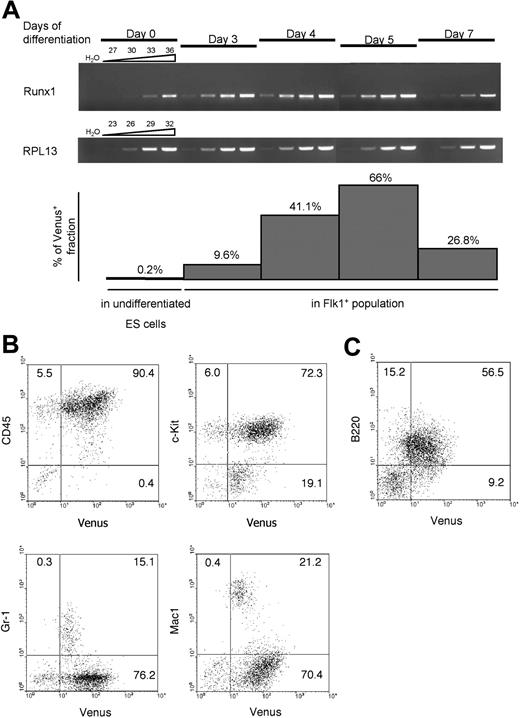
We measured the mRNA levels of endogenous Runx1 during in vitro differentiation of the ES clones (Figure 2A). Runx1 expression was detected on day 3 at a substantially higher level than on day 0, and this preceded the appearance of Venus+ population at day 4. The lag may be explained by rapid expansion of differentiating ES cells from day 0 to day 3, which severely decreased the amount of Venus protein in a single cell to the level of fluorescence not detectable by flow cytometry as Venus+. After day 4, the mRNA levels of Runx1 and the frequency of Venus+ cells in Flk-1+ population correlated well. These data suggest that the expression of Venus reflects the endogenous levels of Runx1. In addition, these Runx1Venus/+ ES lines were tested for specificity of the expression of the introduced reporter. Cultured on OP9 stromal cell layers in the presence of hematopoietic cytokines and VEGF, the recombinant ES cells efficiently differentiated into myeloid and lymphoid lineages. Venus fluorescence was detected in almost all CD45+ hematopoietic cells (Figure 2B). c-Kit+ immature hematopoietic cells and most B220+ lymphocytes expressed a high level of Venus, whereas differentiated Mac-1 and Gr-1+ cells expressed the reporter gene at lower levels (Figure 2B-C). These data suggest that the expression pattern of the Venus reporter gene faithfully reflects the known pattern of Runx1 expression in the hematopoietic lineages.34-36
Venus YFP expression during ES cell differentiation on OP9 stroma. (A) RT-PCR analysis of endogenous Runx1 mRNA transcribed from proximal promoter P2, and corresponding Venus expression in undifferentiated ES cells and in Flk-1+ fraction of differentiated ES cells. Above the open elongated triangles are the numbers of PCR cycles; days of ES cell differentiation on OP9 cell layer are indicated. RPL13 indicates ribosomal protein 13 large subunit. (B) Venus expression in ES cell–derived myeloid hematopoietic cells. (C) Venus expression in ES cell–derived B cells.
Venus YFP expression during ES cell differentiation on OP9 stroma. (A) RT-PCR analysis of endogenous Runx1 mRNA transcribed from proximal promoter P2, and corresponding Venus expression in undifferentiated ES cells and in Flk-1+ fraction of differentiated ES cells. Above the open elongated triangles are the numbers of PCR cycles; days of ES cell differentiation on OP9 cell layer are indicated. RPL13 indicates ribosomal protein 13 large subunit. (B) Venus expression in ES cell–derived myeloid hematopoietic cells. (C) Venus expression in ES cell–derived B cells.
Runx1 expression during differentiation of Flk-1+ cells
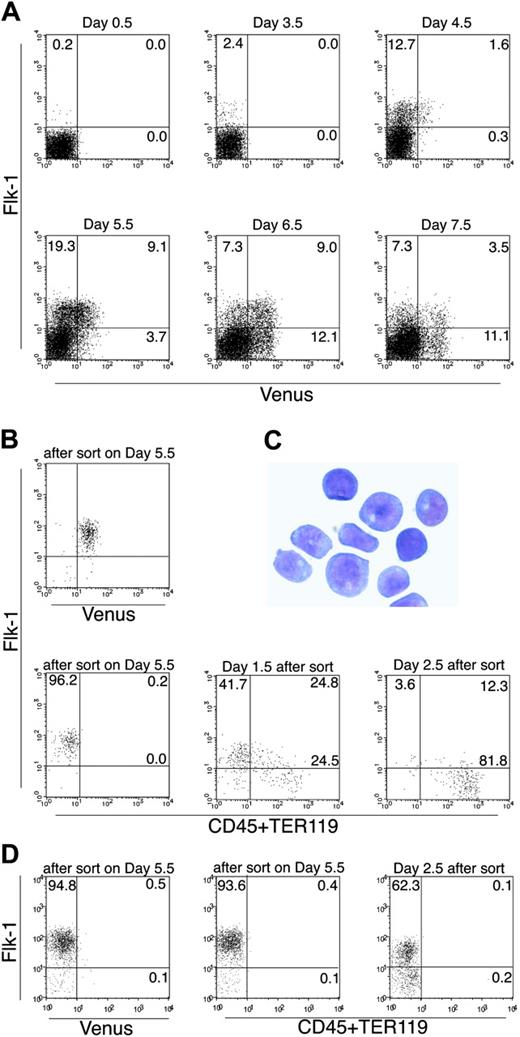
We next investigated Runx1 expression during the differentiation of mesodermal cells and ECs. ES cell differentiation was induced on OP9 stromal cells, which support preferential ES cell differentiation to mesodermal lineages. Like other ES cell lines,16,17 Flk-1+ cells appeared at approximately day 3.5, and the number of the Flk-1+ cells increased within the next 24 hours in the Runx1Venus/+ ES cell (Figure 3A). Runx1-Venus+ cells appeared approximately 1 day later than did the Flk-1+ fraction, and the number of Runx1-Venus+ cells increased in the subsequent 24 hours (from 1.6% to 9.1%). Nearly all Runx1+ cells induced under these conditions were Flk-1+ at this stage. This observation is consistent with the results of a previous study by Lacaud et al.37 In our study, Runx1 expression was also investigated at later stages of differentiation. From day 6.5 to day 7.5, the double-positive cells disappeared quickly and the Flk-1- population became dominant within the Runx1-Venus+ cells (11.1% vs 3.5%, respectively). Considering that Runx1 is a requisite molecule for the differentiation of ECs to HPCs, these data suggest a sequence of events in which Runx1 begins to be expressed in a portion of Flk-1+ cells, in turn triggering the down-regulation of Flk-1 and initiating transition to hematopoietic specification.
To confirm this possibility, we sorted the Flk-1+Venus+ cells on day 5.5 of differentiation and cultured them again on OP9 feeder layers to induce further differentiation (Figure 3B). With the induction of differentiation, the double-positive cells quickly disappeared and eventually differentiated into HPCs (Figure 3B-C). In contrast, when we cultured the Flk-1+Venus- cells sorted from the same culture and incubated with OP9, 30% to 70% of the cells maintained Flk-1 expression and no HPCs were generated (Figure 3D); however, day 4 to 4.5 Flk-1+Venus- cells can form HPCs because some cells at this stage of differentiation still have the potential to up-regulate Runx1-Venus (Figure 3A), but later Flk-1+Venus- fractions are completely devoid of such cells. These findings indicate that a subpopulation of Flk-1+ cells that expresses Runx1 is the sole source of hematopoietic progenitors and that Flk-1 is down-regulated during HPC generation.
FACS analysis of Venus knock-in ES cells differentiating on OP9 stromal cells. (A) Runx1Venus/+ ES cells were cultured on OP9 cells and analyzed for the expression of Flk-1 and Venus by fluorescence-activated cell sorter (FACS) on the indicated days. (B) Flk-1+Venus+ cells were sorted on day 5.5 of differentiation and recultured on OP9 stroma. Expression of Flk-1, CD45, and TER119 on Venus+ cells was then analyzed using FACS on the indicated days. (C) Giemsa staining of Venus+ cells harvested on day 2.5 of differentiation of sorted Flk-1+Venus+ cells. All cells looked like immature hematopoietic cells of definitive hematopoietic origin. The image was captured with an Olympus DP50 microscope (× 40/0.75 NA objective lens) and DP control software (Olympus, Tokyo, Japan). (D) Flk-1+Venus- cells were sorted on day 5.5 of differentiation and cultured again on OP9 stroma. On day 2.5 after sorting, cells were harvested and stained for Flk-1, CD45, and TER119 and were analyzed by flow cytometry. Results shown are representative of 3 independent experiments. Error bars indicate standard deviation of duplicate experiments.
FACS analysis of Venus knock-in ES cells differentiating on OP9 stromal cells. (A) Runx1Venus/+ ES cells were cultured on OP9 cells and analyzed for the expression of Flk-1 and Venus by fluorescence-activated cell sorter (FACS) on the indicated days. (B) Flk-1+Venus+ cells were sorted on day 5.5 of differentiation and recultured on OP9 stroma. Expression of Flk-1, CD45, and TER119 on Venus+ cells was then analyzed using FACS on the indicated days. (C) Giemsa staining of Venus+ cells harvested on day 2.5 of differentiation of sorted Flk-1+Venus+ cells. All cells looked like immature hematopoietic cells of definitive hematopoietic origin. The image was captured with an Olympus DP50 microscope (× 40/0.75 NA objective lens) and DP control software (Olympus, Tokyo, Japan). (D) Flk-1+Venus- cells were sorted on day 5.5 of differentiation and cultured again on OP9 stroma. On day 2.5 after sorting, cells were harvested and stained for Flk-1, CD45, and TER119 and were analyzed by flow cytometry. Results shown are representative of 3 independent experiments. Error bars indicate standard deviation of duplicate experiments.
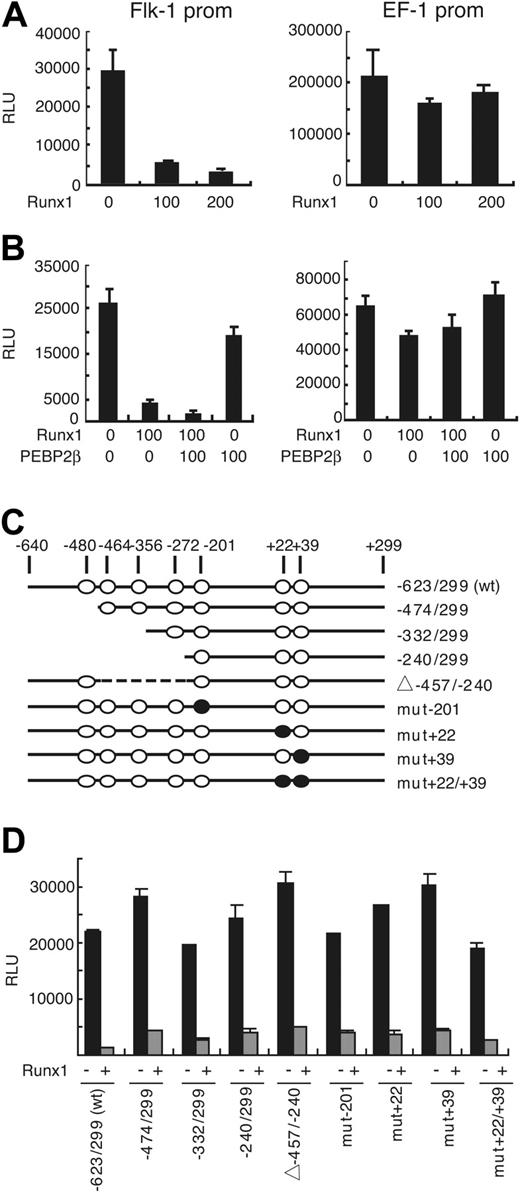
Runx1 down-regulates the activities of the Flk-1 promoter in BAEC. (A) The activity of luciferase reporter gene under the control of the -640 bp/+299 bp promoter fragment of mouse Flk-1 was measured in the presence of the indicated amounts (nanograms) of Runx1 expression vector (left panel). Note that luciferase activity was down-regulated in the presence of Runx1 in a dose-dependent manner, whereas luciferase activity driven by elongation factor (EF-1) promoter was not affected (right panel). (B) The repressive effect of Runx1 on the Flk-1 promoter was augmented in the presence of PEBP2β (left panel), whereas EF-1 promoter activity was not affected (right panel). (C) Putative Runx1-binding sites in the murine Flk-1 promoter and functional analysis of the promoter. (○) Putative Runx1-binding sites. (•) Binding sites mutated by PCR. (D) Activity of luciferase reporter under control of mutant Flk-1 promoters in BAECs. Results shown are representative of 3 independent experiments. RLU indicates relative light unit.
Runx1 down-regulates the activities of the Flk-1 promoter in BAEC. (A) The activity of luciferase reporter gene under the control of the -640 bp/+299 bp promoter fragment of mouse Flk-1 was measured in the presence of the indicated amounts (nanograms) of Runx1 expression vector (left panel). Note that luciferase activity was down-regulated in the presence of Runx1 in a dose-dependent manner, whereas luciferase activity driven by elongation factor (EF-1) promoter was not affected (right panel). (B) The repressive effect of Runx1 on the Flk-1 promoter was augmented in the presence of PEBP2β (left panel), whereas EF-1 promoter activity was not affected (right panel). (C) Putative Runx1-binding sites in the murine Flk-1 promoter and functional analysis of the promoter. (○) Putative Runx1-binding sites. (•) Binding sites mutated by PCR. (D) Activity of luciferase reporter under control of mutant Flk-1 promoters in BAECs. Results shown are representative of 3 independent experiments. RLU indicates relative light unit.
Runx1 represses Flk-1 promoter transcriptional activity in ECs
During HPC differentiation, Runx1 might trigger multiple events, and the rapid down-regulation of Flk-1 in the Flk-1+Runx1+ cells can be either a direct or an indirect outcome of Runx1 expression. To investigate whether Runx1 acts directly on Flk-1 transcription, we first examined Flk-1 promoter activity in the presence or absence of exogenous Runx1. We used BAECs in this assay, because the Flk-1 promoter is active specifically in ECs.25,38
The -640/+299 fragment of the Flk-1 gene was ligated to firefly luciferase cDNA and transiently transfected into BAECs with or without Runx1-expressing plasmid vectors. Flk-1 promoter activity was reduced by 5- to 10-fold in the presence of Runx1 in a dose-dependent manner (Figure 4A). In contrast, Runx1 had no effect on the promoter of elongation factor-1 (EF-1). Runx1-dependent repression of the Flk-1 promoter is EC specific; we could not detect the negative regulatory effects of Runx1 on the Flk-1 promoter in other cell types, including NIH3T3, COS, or HEK293 cells (data not shown).
Runx1 is a member of the PEBP2 transcription complex, and its DNA binding and transcriptional regulation activities are increased by binding with PEBP2β, the heterodimer partner. We evaluated the effects of PEBP2β on the repression of Flk-1 promoter transcription by Runx1. PEBP2β significantly augmented the effect of Runx1 (P < .005), whereas the effect of Runx1 on the activity of the EF-1 promoter was not affected by the addition of PEBP2β (Figure 4B). This further confirms that the Flk-1 gene promoter is a specific target of gene silencing induced by the Runx1-mediated transcription complex.
There are 7 putative consensus-binding sites for Runx1 within the Flk-1 promoter, though not all of them are complete matches. We introduced deletion and point mutations in the sequence of the Flk-1 promoter to disrupt each putative Runx1-binding site and measured the promoter activity of these mutants (Figure 4C-D). Surprisingly, none of the mutants reversed the Runx1-mediated transcriptional repression, suggesting the repressive effects are indirect.
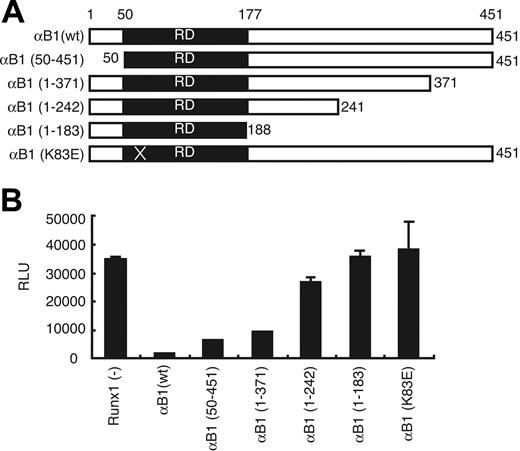
To determine which Runx1 domain is required for this repression, we examined Runx1 deletion mutants in our assay (Figure 5A). Amino acids 242 to 371 were important for this inhibitory effect of Runx1 (Figure 5B). When we transfected BAECs with the Runx1 mutant K83E, which maintains the ability to heterodimerize with the β subunit but loses DNA-binding ability,27 Flk-1 promoter activity was not suppressed. Taken together, these data strongly suggest that Runx1 has negative regulatory effects on Flk-1 promoter activity in ECs, and this repression requires intact DNA-binding and transactivating domains of Runx1 protein.
Overexpression of Runx1 inhibits Flk-1 expression during in vitro differentiation of ES cells
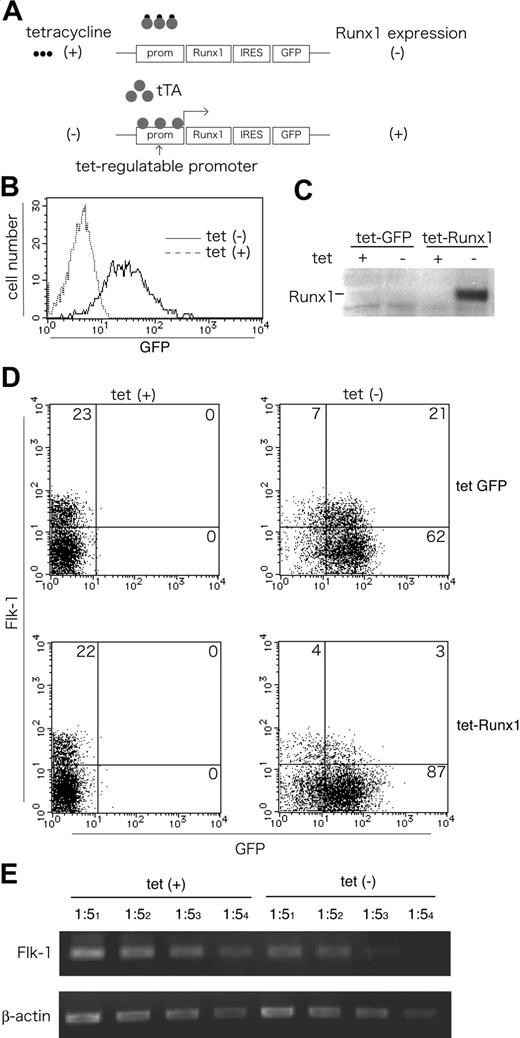
We next investigated whether Runx1-dependent repression of Flk-1 is observed in the ES cell differentiation culture as well as in the EC lines. For this purpose, we used a Tet-off inducible system in combination with in vitro differentiation of ES cells29 (Figure 6A). We generated ES cell lines in which the tTA gene was driven by the CAG promoter and in which Runx1 and/or green fluorescence protein (GFP) expression, was under the control of the Tet operator sequence. Expression of Runx1 or GFP expression was induced in the absence of tetracycline, whereas neither Runx1 nor GFP expression was detected in the presence of tetracycline (Figure 6B-C).
Analysis of Runx1 functional domains involved in the Flk-1 promoter repression. (A) Schematic illustration of the structures of full-length Runx1 (αB1), its deletion derivatives, and K83E mutants. K83E mutant has an A>G substitution in exon 3 resulting in a missense mutation. (B) Effects of Runx1 and its mutants on Flk-1 promoter activity in BAECs. Results shown are representative of 3 independent experiments. RD indicates runt domain.
Analysis of Runx1 functional domains involved in the Flk-1 promoter repression. (A) Schematic illustration of the structures of full-length Runx1 (αB1), its deletion derivatives, and K83E mutants. K83E mutant has an A>G substitution in exon 3 resulting in a missense mutation. (B) Effects of Runx1 and its mutants on Flk-1 promoter activity in BAECs. Results shown are representative of 3 independent experiments. RD indicates runt domain.
Tet-induced overexpression of Runx1 down-regulates Flk-1 in differentiating ES cells. (A) The construct and strategy of Tet-regulated expression of Runx1 in ES cells. The expression of Runx1 and IRES-linked GFP is driven by a Tet-regulated promoter suppressed by the addition of tetracycline (Tet-off system). (B-C) Inducible expression of GFP and Runx1 by the removal of Tet in undifferentiated ES cells. GFP expression was analyzed using FACS (B), and Runx1 expression was analyzed using Western blotting (C) 48 hours after Tet removal. (D-E) Inducible expression of Runx1 down-regulates Flk-1 expression during in vitro differentiation of ES cells. ES cells with Tet-inducible GFP constructs (top lane) or Tet-inducible Runx1-IRES-GFP constructs (bottom lane) were cultured on collagen type 4–coated dishes in the presence or absence of tetracycline and analyzed for Flk-1 and GFP expression by flow cytometry (D) and for Flk-1 expression by semiquantitative RT-PCR (E) on day 4.5 of differentiation. Results shown are representative of 3 independent experiments.
Tet-induced overexpression of Runx1 down-regulates Flk-1 in differentiating ES cells. (A) The construct and strategy of Tet-regulated expression of Runx1 in ES cells. The expression of Runx1 and IRES-linked GFP is driven by a Tet-regulated promoter suppressed by the addition of tetracycline (Tet-off system). (B-C) Inducible expression of GFP and Runx1 by the removal of Tet in undifferentiated ES cells. GFP expression was analyzed using FACS (B), and Runx1 expression was analyzed using Western blotting (C) 48 hours after Tet removal. (D-E) Inducible expression of Runx1 down-regulates Flk-1 expression during in vitro differentiation of ES cells. ES cells with Tet-inducible GFP constructs (top lane) or Tet-inducible Runx1-IRES-GFP constructs (bottom lane) were cultured on collagen type 4–coated dishes in the presence or absence of tetracycline and analyzed for Flk-1 and GFP expression by flow cytometry (D) and for Flk-1 expression by semiquantitative RT-PCR (E) on day 4.5 of differentiation. Results shown are representative of 3 independent experiments.
When we cultured the control cell lines with inducible GFP for 4 days without LIF, Flk-1 expression was detected at equivalent levels in the presence or absence of tetracycline (Figure 6D). In contrast, when we cultured cell lines carrying inducible Runx1 transgene with or without tetracycline, the number of Flk-1–expressing cells was significantly reduced by Runx1 overexpression (3.7% vs 23.2%; P < .001; n = 3). Cell recovery and gross morphology did not differ with or without tetracycline treatment in either clone. This repression of Flk-1 expression was accompanied by a reduction in Flk-1 transcripts (Figure 6E). The same results were obtained in 10 independent ES cell clones, suggesting that Runx1 has the potential to down-regulate the expression of the endogenous Flk-1 gene during differentiation of ES cells in vitro.
Discussion
During the EC–HPC transition, molecules determining the essential features of ECs as an integral part of the endothelial sheet are down-regulated while HPC features are acquired. The budding of HPCs from the dorsal aorta, which regularly occurs in normal embryos, is not detectable in Runx1 null-mutant mice,21 and no hemogenic ECs are detected in the embryonic vessels of the mutant mice.11 These studies strongly suggest that Runx1 is directly involved in the EC–HPC transition. The aim of this study was to dissect this process, with particular focus on the relation between Runx1 and Flk-1.
Under OP9 stromal cell culture conditions, which preferentially support the differentiation of ES cells to a mesodermal lineage, Runx1 is induced mostly within the Flk-1+ population. This finding is largely consistent with the results of a previous study by Lacaud et al,37 except that the time course of Runx1 expression was delayed 24 hours in the present experiment. Although part of this delay reflects the inherent difference between the 2 methods of ES cell differentiation, it is likely that the enhancement of HPC differentiation by haploinsufficiency of Runx1 accounts for this difference in the time course. Indeed, recent work by the same group indicates that Runx1 haploinsufficiency profoundly affects the time course of ES cell differentiation into HPC.33 In contrast, our results indicated that Runx1Venus/+ ES cells differentiated identically to Runx1+/+ ES cells in terms of expression of Flk-1 mesodermal marker, suggesting Runx1 is at least partially intact in the recombinant allele. We are intensively investigating the biologic function of the knock-in allele in vivo.
Runx1 is induced within 24 hours of the appearance of Flk-1+ cells, which is almost simultaneous with the expression of EC markers such as VE cadherin. It is unclear which mechanism is responsible for the divergence of Runx1+ and Runx1- ECs during EC commitment. It has been suggested that the EC–HPC transition occurs in a restricted region of the vascular system12,20,22 ; therefore, it is likely that an as yet unknown extrinsic signal is involved in this divergence. Nonetheless, our study clearly demonstrates that HPCs are generated only from the Flk-1+Runx1+ population. Consistent with our results, the generation of HPCs in midgestation embryos occurred exclusively in the Runx1+ population with or without EC features,20 indicating that the ability to undergo the EC–HPC transition is determined at a relatively early stage of mesoderm differentiation. Such fine-tuning of the timing of Runx1 expression in relation to Flk-1 must be essential for supporting the process wherein HPC progenitor cells are first integrated into the vascular system and subsequently differentiate into HPCs.
In this study, we investigated the fate of Flk-1+Runx1+ cells. Under our culture conditions, most double-positive cells differentiated into HPCs, though a small population that down-regulated Runx1 expression maintained EC characteristics. ECs express Runx1 in vivo in the yolk sac, the vitelline and umbilical arteries, and the ventral wall of the dorsal aorta in the aorta/genital ridge/mesonephros region, where definitive HPCs arise as cell clusters attach to the luminal wall.35 This supports the view that the program leading to Runx1 expression in Flk-1+ cells is likely to trigger the irreversible process toward HPC differentiation and is consistent with our previous report22 that a subset of ECs in which a promoter/enhancer combination of the Flk-1 gene was active, but Runx1 expression was absent, could not give rise to HPCs.
Flk-1 is a VEGF receptor that has a central role in endothelial development.13,14 VEGF–Flk-1 signaling mediates proliferation, migration, and other EC-specific properties. Disruption of Flk-1 resulted in embryonic lethality with no ECs or HPCs in vivo; however, Flk-1-/- ES cells can differentiate into both lineages in vitro,39,40 indicating that Flk-1 is required especially for the migration of progenitors into the proper microenvironment during embryogenesis. Heterozygous inactivation of the VEGF gene results in impaired development of the vascular and hematopoietic systems.41 In chicken, a higher concentration of VEGF inhibits the differentiation of HPCs from VEGF-R2+ cells.42 These data indicate that precise regulation of VEGF signaling is necessary not only for EC development but also for proper HPC development. In this study, we demonstrated that the Runx1+Flk-1+–sorted population quickly down-regulates Flk-1 expression upon reseeding on a fresh OP9 stromal cell layer, suggesting an inhibitory role of Runx1 in the regulation of Flk-1 expression. Indeed, Lacaud et al33 reported that heterozygous inactivation of Runx1 accelerated mesodermal differentiation using an ES in vitro system and that a higher proportion of mesodermal cells than normal ES cells expressed Flk-1. Conversely, we demonstrated that Runx1 specifically represses the promoter activity in a cell context–dependent manner. This repression was also observed during early mesoderm differentiation, through which Flk-1 is induced. Taken together, these data provide evidence that Runx1, possibly among others, is a negative regulator of Flk-1 expression during mesoderm differentiation. It is possible that the tuning of HPC differentiation by the dose of Runx1 is mediated through regulation of the Flk-1 expression level. On one hand, Runx1 down-regulates the signal mediated by VEGF/Flk-1 and thereby inhibits the differentiation toward ECs at the diverging point of cellular specification, while strong VEGF-Flk-1 signaling inhibits HPC differentiation, as Eichmann et al demonstrated.42
The Runx family of transcription factors acts as transcription activators and as transcriptional repressors or silencers, depending on the cellular context and target molecules. The mechanisms of repression by the Runx family have been studied in detail.43 The recruitment of corepressors, including mSin3A, TLE, SUV39H1, and histone deacetylase, is also involved in Runx1-mediated repression.43-47 In our study, disruption of the VWRPY motif had little impact on repression, suggesting that TLE is not involved in this effect (αB1 [1-371] deletion mutant; Figure 5). Although our results with the Runx1 K83E mutant clearly showed involvement of DNA binding in the inhibitory function of Runx1, we could not determine the elements in Flk-1 promoter responsible for repression in a reporter assay, suggesting Runx1 transcription factor targets another gene in the chain of events leading to down-regulation of Flk-1. Hypoxia-inducible factor-1 (HIF-1) and HIF-2 positively regulate the Flk-1 promoter, and the binding sites for the SCL, GATA, and Ets transcription factors have important roles in the proper expression of Flk-1 during development in vivo.48,49 Whether these factors are directly silenced by Runx1 in the described inhibition of Flk-1 expression remains to be determined.
Our results showed that amino acids 242 to 371, known as a transactivating domain, are important for repression. These findings prompted us to study the role of the bone morphogenetic protein (BMP)–Smad signaling pathway because BMP4 is also involved in the differentiation of hematopoietic and endothelial lineages50 and Smads family member molecules are known to interact with Runx1 at its transactivating domain.51 When Runx1 expression vector was cotransfected with expression constructs for Smad1, Smad5, or Smad8, together with a vector for a constitutively active form of the BMP receptor, the repressive effects of Runx1 were slightly attenuated, suggesting that Smads were not involved in this repression by Runx1 but rather sequestered Runx1 from the repressive effects (data not shown).
In summary, we have demonstrated that Runx1 represses Flk-1 expression during the development of definitive hematopoiesis. These findings provide new insight into the understanding of diversification between hematopoietic and endothelial lineages.
Prepublished online as Blood First Edition Paper, May 31, 2005; DOI 10.1182/blood-2004-12-4872.
Supported by grant 12219209 from the Ministry of Education and Science of Japan (S.-I.N.) and by the Project for Realization of Regenerative Medicine (S.-I.N.). I.M.S. was supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Motomi Osato, Dr Takumi Era, and Dr Georg Breier for providing Runx1 expression vectors, vectors for the tet-regulatable system, and pGLacZ-Flk-1 promoter/enhancer, respectively. We also thank Dr Gang Huang, Dr Jun Yamashita, and Dr Osam Mazda for valuable discussions and Natalia I. Samokhvalova for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal