Abstract
This study was undertaken to analyze the differentiation profiles assessed by immunophenotyping in AIDS-related B-cell lymphoma (ARL) and their relation to the clinical course. Paraffin-embedded sections of 89 ARL cases during 1989 to 2004 were stained immunohistochemically with antibodies to CD3, CD10, CD20, CD38, CD138/Syndecan-1 (Syn-1), multiple myeloma-1/interferon regulatory factor-4 (MUM1/IRF4), B-cell lymphoma protein-2 (BCL-2), BCL-6, latent membrane protein-1 (LMP-1), and Ki-67. Expression of CD10 and CD20 were associated with better overall survival (OS; P = .009 and P = .04, respectively). Expression of CD20 was associated with longer disease-free survival (DFS; P = .03), whereas expression of CD138/Syn-1 was associated with shorter DFS (P = .03). OS and DFS were worse in patients with immunophenotypic profiles related to post-germinal center (GC) differentiation (BCL-6 and CD10 negative, MUM1/IRF4 and/or CD138/Syn-1 positive) when compared with GC differentiation (P = .01). When controlled for age-adjusted International Prognostic Index (IPI), prior AIDS-defining illness (ADI), and year of ARL diagnosis, a post-GC differentiation remained significantly associated with poor OS and DFS. Expression of CD10 was associated with a preserved immunocompetence, whereas CD20 was less frequent in patients developing ARL while on highly active antiretroviral therapy (P = .04). In summary, lack of CD20 or CD10 expression and a post-germinal center signature are associated with a worse prognosis in ARL. (Blood. 2005;106:1762-1769)
Introduction
In patients infected with HIV-1, AIDS-related lymphoma (ARL) remains one of the most frequent AIDS-defining illnesses (ADIs). Epidemiologic data suggest that since the introduction of highly active antiretroviral therapy (HAART), the decrease of the incidence of ARL has been less profound than that seen with other ADIs.1-3 ARL shares some distinctive features when compared with non-Hodgkin lymphoma (NHL) in the general population. This includes not only an almost exclusive B-cell derivation, a propensity toward advanced and extranodal disease, but also clinical aggressiveness.4,5 Despite these particular characteristics, ARL is a biologically heterogeneous disease. The different subtypes of ARL vary substantially with regard to several factors: Epstein-Barr virus and other viral infections, the extent of immune deficiency, oncogenic mutations, cytokine dysregulation, and histogenetic derivation.6-8 However, to date several studies failed to demonstrate an association between distinct histologic subtypes of ARL and prognosis.9-15
For HIV-negative NHL, gene expression profiling using complementary DNA (cDNA) microarrays revealed that current taxonomy of lymphoma includes very heterogenous diseases. For example, distinct forms of diffuse large B-cell lymphoma (DLBCL) exist with gene expression patterns indicative of different stages of B-cell differentiation and activation.16,17 The molecular differences in the cDNA microarray technique are accompanied by a remarkable divergence in clinical behavior, reflected in a much-improved prognosis for patients with a germinal center B-like type.16 Immunohistochemical analysis on paraffin-embedded tissues is more readily available and less cost intensive than cDNA microarray and provides similar prognostic information.18 Indeed, expression of immunohistochemical markers for germinal center differentiation such as B-cell lymphoma protein-6 (BCL-6) or CD10 has been found to be predictive for favorable outcome or particular clinicopathologic features in many studies on DLBCL18-24 and also in specific entities such as cutaneous DLBCL or mucosa-associated lymphoid tissue (MALT) lymphoma.25,26 In contrast, the expression of markers indicating activation of peripheral blood B cells such as multiple myeloma-1/interferon regulatory factor-4 (MUM1/IRF4) or proto-oncogenes such as BCL-2 has been shown to have an independent adverse effect in HIV-negative NHL.18,21,23,27-30
In ARL, data on immunohistochemical expression profiles suggest distinct categories that cluster between histogenetic subtypes.31,32 The clinical value of the histogenetic derivation patterns, however, has yet to be elucidated. In addition, data on changes in expression profiles over time are lacking. In this study, we investigated the immunohistochemical expression throughout the pathologic spectrum of AIDS-related B-cell lymphomas using a broad panel of expression and proliferation markers. Expression profiles were correlated with both clinical data and clinical course and were analyzed for changes over time.
Patients, materials, and methods
Patients
This cohort consisted of 89 patients infected with HIV-1 with systemic high-grade ARL, diagnosed histologically between 1989 and 2004 in 3 pathology centers (St Georg Hospital, Hamburg, 36 cases; University of Kiel, 30 cases; University of Cologne, 23 cases). Baseline characteristics and ARL features are shown in Table 1. Cases had been identified by the computed database of the corresponding HIV centers located at the same institution and were selected based on the availability of paraffin-embedded tissue suitable for immunohistochemistry. Approval was obtained from the University of Kiel Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. In all patients data were evaluated with regard to demographic characteristics, HIV- and HAART-related data, ARL staging according to Ann Arbor classification,33 treatment, and remission stage of ARL according to standardized guidelines.34 As only 3 of 89 patients were older than 60, the age-adjusted International Prognostic Index (IPI)35 was calculated based on tumor stage, lactate dehydrogenase (LDH) level, and performance status. Since one or more prognostic factors were lacking in some patients, only 2 IPI groups were created for further analysis: a low-risk group (IPI 0 or 1, low and low-intermediate risk) and a high-risk group (IPI 2 or 3, high-intermediate and high risk). Chemotherapy regimens consisting of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), comparable or more intensive regimens were considered to be curative. HAART was defined as a combination of at least 3 antiretroviral drugs. At the last follow-up on June 30, 2004, 57 of 89 patients had died, 7 were lost to follow-up, and 25 were alive. Of the 57 patients who were dead, ARL was considered to be the main cause of death in 31 cases. Fourteen patients died of ADIs. In 4 patients, it was not possible to clearly distinguish between ARL and other ADIs as leading cause of death. Five patients died of unknown reasons but without evidence of ARL recurrence. One patient died of a secondary neoplasia (CD30+ anaplastic T-cell lymphoma that occurred more than 5 years after the initial diagnosis of Burkitt lymphoma), 1 patient died of esophageal carcinoma, and 1 patient committed suicide.
Baseline characteristics at the time of ARL diagnosis
Characteristics . | Data . |
|---|---|
| No. patients studied | 89 |
| No. men/no. women | 82/7 |
| Age, y, mean ± SD (range) | 40.9 ± 9.9 (23.0-69.1) |
| Localization of ARL, no. (%) | |
| At least 1 extranodal manifestation | 63 (71) |
| Strictly nodal manifestation | 26 (29) |
| Ann Arbor stage, no. (%) | |
| I-II | 25 (28) |
| III-IV | 61 (69) |
| No staging available | 3 (3) |
| B symptoms | 53 (63) |
| Modified age adjusted IPI score, no. (%) | |
| Low IPI of 0 or 1 | 28 (31) |
| High IPI of 2 or 3 | 51 (57) |
| No IPI available | 10 (11) |
| Immunologic, virologic, and other parameters | |
| Prior AIDS diagnosis, no. (%) | 27 (31) |
| Absolute CD4 cell counts, cells/μL, mean ± SD (range) | 201 ± 198 (0-960) |
| Viral load, log copies/mL, mean ± SD (range) | 4.57 ± 1.45 (0.90-6.43) |
| Elevated LDH, no. (%) | 53 (65) |
| Hemoglobin level, g/dL, mean ± SD (range) | 12.2 ± 2.2 (7.2-17.3) |
Characteristics . | Data . |
|---|---|
| No. patients studied | 89 |
| No. men/no. women | 82/7 |
| Age, y, mean ± SD (range) | 40.9 ± 9.9 (23.0-69.1) |
| Localization of ARL, no. (%) | |
| At least 1 extranodal manifestation | 63 (71) |
| Strictly nodal manifestation | 26 (29) |
| Ann Arbor stage, no. (%) | |
| I-II | 25 (28) |
| III-IV | 61 (69) |
| No staging available | 3 (3) |
| B symptoms | 53 (63) |
| Modified age adjusted IPI score, no. (%) | |
| Low IPI of 0 or 1 | 28 (31) |
| High IPI of 2 or 3 | 51 (57) |
| No IPI available | 10 (11) |
| Immunologic, virologic, and other parameters | |
| Prior AIDS diagnosis, no. (%) | 27 (31) |
| Absolute CD4 cell counts, cells/μL, mean ± SD (range) | 201 ± 198 (0-960) |
| Viral load, log copies/mL, mean ± SD (range) | 4.57 ± 1.45 (0.90-6.43) |
| Elevated LDH, no. (%) | 53 (65) |
| Hemoglobin level, g/dL, mean ± SD (range) | 12.2 ± 2.2 (7.2-17.3) |
ARL indicates AIDS-related lymphoma; IPI, International Prognostic Score; LDH, lactate dehydrogenase.
Baseline characteristics. The median age of the patients was 39.8 years. The cohort included 85 whites and 4 African Americans living in Germany. The most frequent mode of HIV-1 infection was male-to-male sexual transmission (71%). Few patients reported heterosexual transmission (6%) or intravenous drug use (6%), came from pattern II countries (3%), or were hemophiliac (2%). In 12%, the mode of infection was unknown, or data were unavailable. The median time from the first positive HIV test to ARL diagnosis was 4.2 years. Profound immunosuppression at ARL diagnosis was a common finding, with only 39% having a CD4 cell count above 200 cells/μL (median CD4 cell count of 150/μL). Of 43 patients with an evaluable plasma viremia at ARL diagnosis, only 7 (16%) had a viral load of fewer than 500 copies/mL. Extranodal manifestation was a common feature as was advanced stage and an elevated LDH.
Chemotherapy. Following ARL diagnosis, 15 patients (17%) received no treatment (n = 13) or only palliative chemotherapy (n = 2). A curative chemotherapy was initiated in 74 (83%) of 89 patients which consisted of CHOP-based regimens in 66 of 74 patients (in 8 patients CHOP was intensified with etoposide). Eight patients received a more-intensive regimen that was adapted from the German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia (GMALL)36 and was intensified by rituximab in one patient. This was the only patient who received an additional therapy with monoclonal antibodies. A complete remission (CR) was achieved in 70% of patients starting curative chemotherapy.
Antiretroviral therapy. Before the diagnosis of ARL, 48 of the patients (54%) were naive to antiretroviral therapy (ART) and 25 (28%) had had mono- or dual-ART. Fifteen patients (17%) had received HAART for a median of 1.44 years (range, 0.31-6.73 years, 5 patients had been treated for less than 1 year), among them 80% (12 of 15) a protease inhibitor-based HAART. In one patient data on ART history was insufficient. During the first 2 years after diagnosis of ARL, 32 patients (36%) received no ART, 14 patients (16%) were treated with mono- or dual-ART, and 38 patients (43%) were on HAART. Five patients (6%) had insufficient data or follow-up was insufficient. Of the 32 patients on HAART after diagnosis of ARL during 1997 to 2004, almost all (89%) achieved at least one viral load below 500 copies/mL during follow-up.
Histology
All histologic slides were reviewed by 2 experienced hematopathologists (D.J. and M.T.). The diagnosis of ARL was based on the criteria according to the World Health Organization (WHO) classification.37 For each case, hematoxylin-eosin- and Giemsa-stained slides were studied. ARL were all of B-cell origin, indicated by a positive staining for CD20 or CD79a or heavy chain immunoglobulin rearrangement according to standard procedures.38
Within the WHO classification, 58 (65%) of 89 cases were classified as DLBCL, including 6 cases of immunoblastic lymphoma and 11 cases that displayed morphologic features of immunoblastic large-cell lymphomas and that were later diagnosed immunohistochemically as plasmablastic lymphoma by expression of CD38 and negativity for CD20. Beside the DLBCL, 27 (30%) of 89 cases were classified as Burkitt- or Burkitt-like lymphoma (BL/BLL). Of the remaining 4 cases (5%), 1 case was classified as an undifferentiated plasmocytoma. Three cases could not be classified definitely. However, all 3 displayed, albeit unassociated with body cavity effusions, morphologic and immunohistochemical features of primary effusion lymphomas (PELs).37 These cases were probably referable to extracavitary PEL, which has been shown to apparently represent part of the spectrum of PEL.39,40 At least 2 of these cases were associated with HHV-8 infection and/or Kaposi sarcoma, and 2 lymphomas were located within the gastrointestinal tract.
Immunohistochemistry
Immunostainings were performed on 5-μm-thick serial sections from paraffin blocks from formalin-fixed biopsy specimens. Slides from each block were immunostained with antibodies to CD3, CD10, CD20 (if negative, also for CD79a), CD38, CD138/Syndecan-1 (Syn-1), BCL-6, MUM1/IRF4, BCL-2, LMP-1 (latent membrane protein-1), and Ki-67. Table 2 details the antibodies and the immunohistochemical conditions used. Antigen retrieval was achieved by boiling the sections immersed in 0.01 M citric acid, pH 6.0, for 2 minutes 30 seconds in a pressure cooker.42 The immunoreactions were enhanced either by means of the avidin-biotin complex technique43 or the alkaline phosphatase-anti-alkaline phosphatase (APAAP) method44 followed by brief counterstaining with Mayer hematoxylin.
Antibodies used for immunohistochemical analysis
Antibody . | Source . | Antigen retrieval (pH) . | Dilution . |
|---|---|---|---|
| CD3 | Novocastra, Newcastle, United Kingdom | Citrate acid, 0.01 M, (6.0) | 1:20 |
| CD10 | Novocastra, Newcastle, United Kingdom | Citrate acid, 0.01 M, (6.0) | 1:10 |
| CD20 | Dako, Hamburg, Germany | Citrate acid, 0.01 M, (6.0) | 1:5 |
| CD38 | Novocastra, Newcastle, United Kingdom | Citrate acid, 0.01 M, (6.0) | 1:100 |
| CD79 | Dako, Hamburg, Germany | Citrate acid, 0.01 M, (6.0) | 1:200 |
| MUM1/IRF4 | Dako, Hamburg, Germany | Citrate acid, 0.01 M, (6.0) | 1:50 |
| CD138/Syn-1 | Dako, Hamburg, Germany | Citrate acid, 0.01 M, (6.0) | 1:20 |
| BCL-2 | Zytomed, Berlin, Germany | Citrate acid, 0.01 M, (6.0) | 1:50 |
| LMP-1 | Dako, Hamburg, Germany | TE buffer, (9.0) | 1:200 |
| BCL-6 | Dako, Hamburg, Germany | Target Retrieval Solution, (9.9) | 1:10 |
| Ki-67 | Hematopathology, Kiel, Germany41 | Citrate acid, 0.01 M, (6.0) | 1:10 |
Antibody . | Source . | Antigen retrieval (pH) . | Dilution . |
|---|---|---|---|
| CD3 | Novocastra, Newcastle, United Kingdom | Citrate acid, 0.01 M, (6.0) | 1:20 |
| CD10 | Novocastra, Newcastle, United Kingdom | Citrate acid, 0.01 M, (6.0) | 1:10 |
| CD20 | Dako, Hamburg, Germany | Citrate acid, 0.01 M, (6.0) | 1:5 |
| CD38 | Novocastra, Newcastle, United Kingdom | Citrate acid, 0.01 M, (6.0) | 1:100 |
| CD79 | Dako, Hamburg, Germany | Citrate acid, 0.01 M, (6.0) | 1:200 |
| MUM1/IRF4 | Dako, Hamburg, Germany | Citrate acid, 0.01 M, (6.0) | 1:50 |
| CD138/Syn-1 | Dako, Hamburg, Germany | Citrate acid, 0.01 M, (6.0) | 1:20 |
| BCL-2 | Zytomed, Berlin, Germany | Citrate acid, 0.01 M, (6.0) | 1:50 |
| LMP-1 | Dako, Hamburg, Germany | TE buffer, (9.0) | 1:200 |
| BCL-6 | Dako, Hamburg, Germany | Target Retrieval Solution, (9.9) | 1:10 |
| Ki-67 | Hematopathology, Kiel, Germany41 | Citrate acid, 0.01 M, (6.0) | 1:10 |
Tonsil samples from routine files were used both as positive and negative controls (the latter being incubated with serum instead of the primary antibody). The immunohistochemical staining was considered as positive if 20% or closed sheets of tumor cells showed a positive staining. For the analysis of CD138/Syn-1 and MUM1/IRF4, a cut off of 50% was considered to be positive. For Ki-67 analysis, in each case nuclei from 500 neoplastic cells from randomly selected fields were counted, and the Ki-67 index was calculated as the percentage of positive cells. All cases were reviewed by 2 pathologists (M.T. and D.J.) without knowledge of the clinical data.
Statistics
Fisher exact text was used for comparison of frequencies. The Mann-Whitney U test was used for unpaired comparison of continuous variables. A P value less than .05 was considered to be statistically significant. Kaplan-Meier survival statistics were used to evaluate overall survival (OS) and disease-free survival (DFS). Overall survival was defined as the period from ARL diagnosis to death from any cause or the last contact (which was censored). To reduce the possible bias through confounding by indication resulting from association between patient's pretreatment condition and physician's treatment decision, only patients starting chemotherapy in a curative attempt were included in the univariate and multivariate analysis of OS. DFS was defined as survival without evidence of disease in patients achieving CR. Relapses or deaths from any cause were considered as events. The Cox proportional hazards model was used to evaluate the prognostic value of single immunohistochemical expression markers (CD10, CD20, CD38, CD138/Syn-1, BCL-6, MUM1/IRF4, BCL-2) and/or expression profiles (according to Chang et al20 ) on OS and DFS. Besides these expression markers or profiles, other covariables such as sex, CD4 cell counts (< 100 CD4 cells/μL), a prior AIDS event, a low IPI, and the presence of EBV infection, indicated by the presence of LMP-1, were considered as potential predictors. We also considered the time of ARL diagnosis (early = 1989-1996 versus recent = 1997-2004) as a potential predictor as this reflected best the availability and use of HAART in the patients. Entry criteria for the multivariable Cox regression analysis was a P value less than .1 in univariate analysis. Prognostic factors were identified using forward selection. All analyses were performed with the statistical program StatView (Version 5.01; SAS Institute, Cary, NC).
Results
Clinical characteristics and outcome
In the univariate analysis, several clinical characteristics were associated with an improved OS. An improved OS was observed in patients without a previous ADI versus patients with a previous ADI (P = .01), in patients with a normal LDH level versus an elevated LDH level (P = .02), and in patients with Ann Arbor stage I/II versus stage III/IV (P = .035). LDH and tumor stage were included in the age-adjusted IPI. A low IPI score was significantly associated with an improved OS when compared with a high IPI score (P = .001). No bone marrow involvement (P = .025) and no more than one extranodal manifestation (P = .01) were also associated with increased OS. No differences were observed regarding sex or histology (DLBCL versus BL/BLL versus others). Neither was CD4 cell count at ARL diagnosis predictive, although there was a trend toward a poorer OS in patients with an absolute number of fewer than 100 CD4 cells/μL (P = .09). When analyzing differences between the time intervals 1989 to 1996 and 1997 to 2004, OS was significantly improved within the later period (P = .025). With regard to DFS, a low IPI (P = .01) and no more than one extranodal manifestation (P = .001) were significantly associated with a better outcome. DFS showed no significant difference between the 2 time intervals.
Immunohistochemical expression markers and clinical characteristics
The results of the immunohistochemical analysis and the frequencies of the expression of single markers are shown in Table 3. Representative examples of positive or negative immunohistochemical staining of single markers are depicted in Figure 1A-H. As shown in Table 3, marked differences exist between the different histologic subtypes. For example, whereas the vast majority of DLCBL (immunoblastic and plasmablastic lymphoma excluded) and of BL/BLL showed a CD20 expression, in the remaining cases CD20 expression was rarely seen. Of the 22 cases that stained negative for CD20, 86% (19 of 22) expressed CD38, 85% (17 of 20) expressed MUM1/IRF4, and 52% (11 of 21) expressed CD138/Syn-1. In contrast, only 24% (5 of 21) of cases which stained negative for CD20 expressed CD10, 14% (3 of 22) expressed BCL-2, and 14% (3 of 22) expressed BCL-6. In contrast to markers such as CD10, CD20, and BCL-6, the expression of markers indicating a post-germinal center signature such as CD138/Syn-1 or MUM1/IRF4 was a rare event in DLBCL and BL/BLL cases. In contrast, a high number of the remaining cases stained positive for CD138/Syn-1 or MUM1/IRF4.
Expression frequency of single markers within the cohort and histopathologic subgroups
. | All, % (no./no. total) . | DLBCL, % (no./no. total)* . | BL/BLL, % (no./no. total) . | Others, % (no./no. total)† . | P‡ . |
|---|---|---|---|---|---|
| CD3 | 0 (0/89) | 0 (0/41) | 0 (0/27) | 0 (0/21) | — |
| CD10 | 49 (43/88) | 41 (17/41) | 81 (22/27) | 20 (4/20) | < .001 |
| CD20 | 75 (67/89) | 98 (40/41) | 89 (24/27) | 14 (3/21) | < .001 |
| BCL-6 | 65 (57/88) | 83 (34/41) | 78 (21/27) | 10 (2/20) | < .001 |
| CD38 | 48 (42/88) | 29 (12/41) | 52 (14/27) | 80 (16/20) | < .001 |
| MUM1/IRF4 | 45 (34/75) | 29 (10/35) | 36 (8/22) | 89 (16/18) | < .001 |
| CD138/Syn-1 | 15 (12/80) | 3 (1/36) | 4 (1/26) | 56 (10/18) | < .001 |
| LMP-1 | 20 (17/83) | 20 (8/41) | 12 (3/26) | 38 (6/16) | .126 |
| BCL-2 | 20 (18/89) | 29 (12/41) | 15 (4/27) | 14 (2/21) | .131 |
. | All, % (no./no. total) . | DLBCL, % (no./no. total)* . | BL/BLL, % (no./no. total) . | Others, % (no./no. total)† . | P‡ . |
|---|---|---|---|---|---|
| CD3 | 0 (0/89) | 0 (0/41) | 0 (0/27) | 0 (0/21) | — |
| CD10 | 49 (43/88) | 41 (17/41) | 81 (22/27) | 20 (4/20) | < .001 |
| CD20 | 75 (67/89) | 98 (40/41) | 89 (24/27) | 14 (3/21) | < .001 |
| BCL-6 | 65 (57/88) | 83 (34/41) | 78 (21/27) | 10 (2/20) | < .001 |
| CD38 | 48 (42/88) | 29 (12/41) | 52 (14/27) | 80 (16/20) | < .001 |
| MUM1/IRF4 | 45 (34/75) | 29 (10/35) | 36 (8/22) | 89 (16/18) | < .001 |
| CD138/Syn-1 | 15 (12/80) | 3 (1/36) | 4 (1/26) | 56 (10/18) | < .001 |
| LMP-1 | 20 (17/83) | 20 (8/41) | 12 (3/26) | 38 (6/16) | .126 |
| BCL-2 | 20 (18/89) | 29 (12/41) | 15 (4/27) | 14 (2/21) | .131 |
Total number tested was 89; for DLBCL, n = 41; for BL/BLL, n = 27; and for others, n = 21.
DLBCL indicates diffuse large B-cell lymphoma; BL/BLL, Burkitt and Burkitt-like lymphoma; and —, not applicable.
From the DLBCL, immunoblastic and plasmablastic lymphoma were excluded.
This group included in 6 immunoblastic, 11 plasmablastic lymphoma, 1 undifferentiated plasmocytoma, and 3 cases that displayed morphologic features of primary effusion lymphomas.
Chi-square test, comparing frequencies of markers in the 3 histopathologic subgroups.
We also found some associations between single expression markers and clinical characteristics. Expression of CD10 was significantly associated with a relatively preserved immunocompetence at ARL diagnosis, reflected by a higher proportion of patients with CD4 counts more than 100/μL (P = .008) and with no previous ADI (P = .006). Expression of CD20 was associated with an elevated LDH at ARL diagnosis (P = .03). No other significant associations between expression profile and clinical data were found.
The median percentage of Ki-67-expressing cells was 84%, with a slightly higher percentage in cases that expressed CD10 compared with cases without CD10 expression (89% versus 80%, P = .09). A trend toward a higher percentage was also seen in cases expressing CD20 when compared with cases without CD20 expression (86% versus 80%, P = .11). No other trends were observed between the frequency of Ki-67-expressing cells and other markers.
Expression profiles did not change over time when the time periods between 1989 to 1996 and 1997 to 2004 were compared. No trend could be demonstrated, irrespective whether other/smaller time intervals were chosen. However, analysis of the subgroup of patients developing ARL despite the use of HAART (n = 15; median time on HAART at ARL diagnosis, 1.44 years; 12 of 15 of the patients received a protease inhibitor-based regimen) revealed a high proportion (7 of 15, 47%) of ARL cases without CD20 expression. In contrast, ARL without CD20 expression occurred in only 19% (14 of 73) of patients who were not treated with HAART. This difference was statistically significant (P = .04). Even in the subgroup of patients who developed ARL while having a controlled HIV infection on HAART (HIV plasma viremia of less then 500 copies/mL, n = 7), remained a trend toward cases without CD20 expression (P = .06).
Immunohistochemical staining, APAAP method. (A) DLBCL, centroblastic subtype, CD20 staining with positive tumor cells, × 40; (B) Burkitt-like lymphoma, CD20 staining with negative tumor cells and positive reactive lymphocytes, × 40; (C) DLBCL, centroblastic subtype, CD10 staining with positive tumor cells, × 40; (D) plasmablastic lymphoma, CD10 staining with negative tumor cells, × 40; (E) DLBCL, immunoblastic subtype, CD138 staining with positive tumor cells, × 40; (F) DLBCL, CD138 staining with negative tumor cells and positive reactive plasma cells, × 40; (G) Burkitt lymphoma, MUM1 staining with positive tumor cells, × 40; (H) DLBCL, centroblastic subtype, MUM1 staining with negative tumor cells and positive reactive plasma cells, × 40. Images were visualized in Zeiss Immersol S18N under an Axioskop 2 Plus microscope (Zeiss, Oberkochen, Germany) equipped with a Plan Apochromat 40 ×/1.0 oil objective lens; the iris aperture was 1.0. A Sony 3 CCD color video camera MC3289 (Sony, Tokyo, Japan) and DSH Bilddatenbank software (Greifenstein, Germany) were used for image acquisition.
Immunohistochemical staining, APAAP method. (A) DLBCL, centroblastic subtype, CD20 staining with positive tumor cells, × 40; (B) Burkitt-like lymphoma, CD20 staining with negative tumor cells and positive reactive lymphocytes, × 40; (C) DLBCL, centroblastic subtype, CD10 staining with positive tumor cells, × 40; (D) plasmablastic lymphoma, CD10 staining with negative tumor cells, × 40; (E) DLBCL, immunoblastic subtype, CD138 staining with positive tumor cells, × 40; (F) DLBCL, CD138 staining with negative tumor cells and positive reactive plasma cells, × 40; (G) Burkitt lymphoma, MUM1 staining with positive tumor cells, × 40; (H) DLBCL, centroblastic subtype, MUM1 staining with negative tumor cells and positive reactive plasma cells, × 40. Images were visualized in Zeiss Immersol S18N under an Axioskop 2 Plus microscope (Zeiss, Oberkochen, Germany) equipped with a Plan Apochromat 40 ×/1.0 oil objective lens; the iris aperture was 1.0. A Sony 3 CCD color video camera MC3289 (Sony, Tokyo, Japan) and DSH Bilddatenbank software (Greifenstein, Germany) were used for image acquisition.
Immunohistochemical expression markers and outcome
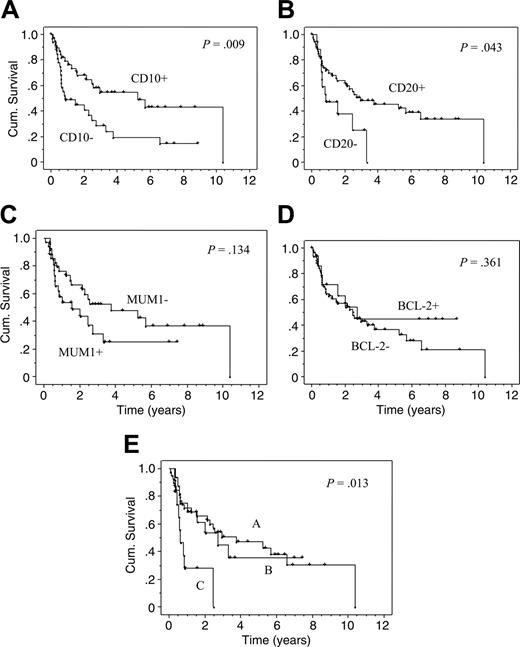
Testing expression markers for their predictive value for OS, a significant benefit for CD10 expression was found in the univariate analysis. The estimated median OS in cases with CD10 expression was 5.3 versus 0.9 years in cases without CD10 expression (P = .009). The difference remained significant when only DLBCL cases were analyzed, even after the exclusion of immunoblastic and plasmablastic subtypes (P = .01). A significantly improved OS was also observed for CD20 expression. The estimated median OS in cases with CD20 expression was 2.9 years versus 0.9 years in cases without CD20 expression (P = .04). Kaplan-Meier curves for OS for both markers, CD10 and CD20, are depicted in Figure 2A-B.20 Although a trend was seen toward an improved OS in patients with ARL staining positive for BCL-6 or with negative staining for CD38, MUM1/IRF4 (Figure 2C) or CD138/Syn-1, these differences did not reach statistical significance.
Kaplan-Meier curves (univariate analysis). Kaplan-Meier curves (univariate analysis) for cases with or without CD10 expression (A), CD20 expression (B), MUM1/IRF4 expression (C), BCL-2 expression (D), and for the 3 patterns according to Chang et al20 (E). In this model, pattern A indicates a GC B-cell pattern (expression of GC B-cell markers such as CD10 and/or BCL-6 but not of activation markers such as MUM1/IRF4 or CD138/Syn-1). Pattern B indicates an activated GC B-cell pattern (expression of at least one GC B-cell marker and of one activation marker). Pattern C indicates an activated non-GC B-cell pattern (expression of MUM1/IRF4 and/or CD138/Syn-1 but not of GC B-cell markers). For all Kaplan-Meier statistics, only patients with a curative chemotherapy were analyzed (n = 74).
Kaplan-Meier curves (univariate analysis). Kaplan-Meier curves (univariate analysis) for cases with or without CD10 expression (A), CD20 expression (B), MUM1/IRF4 expression (C), BCL-2 expression (D), and for the 3 patterns according to Chang et al20 (E). In this model, pattern A indicates a GC B-cell pattern (expression of GC B-cell markers such as CD10 and/or BCL-6 but not of activation markers such as MUM1/IRF4 or CD138/Syn-1). Pattern B indicates an activated GC B-cell pattern (expression of at least one GC B-cell marker and of one activation marker). Pattern C indicates an activated non-GC B-cell pattern (expression of MUM1/IRF4 and/or CD138/Syn-1 but not of GC B-cell markers). For all Kaplan-Meier statistics, only patients with a curative chemotherapy were analyzed (n = 74).
A significantly improved DFS was seen with CD20 (P = .03) but not with CD10 expression. A trend toward a better DFS was also seen for BCL-6 expression (P = .09). In contrast, CD138/Syn-1 expression was significantly associated with poor DFS (P = .03). These results did not change significantly when patients who died because of an AIDS-defining illness without evidence of ARL recurrence were censored (data not shown). Other staining patterns showed no significant correlations with DFS. BCL-2 or LMP-1 staining pattern had neither effect on OS (for BCL-2, see Figure 2D) or DFS in the whole cohort or in any subgroups tested.
Immunohistochemical expression profiles
To analyze the immunohistochemical expression profiles, we first used the histogenetic model for ARL proposed in this journal.31,32 Using 3 markers, namely BCL-6, MUM1/IRF4, and CD138/Syn-1, this model distinguishes between 3 major phenotypic patterns (A, B, C) that display preferential associations with specific clinicopathologic categories. Pattern A reflects GC centroblasts and MUM1/IRF4-negative centrocytes (expression of BCL-6 only) and is associated with BL and DLBCL. Pattern B reflects late GC/early post-GC B cells (expression of MUM1/IRF4 only) and is associated with immunoblastic ARL. Pattern C reflects post-GC B cells (expression of both MUM1/IRF4 and CD138/Syn-1) and is strongly associated with PEL and plasmablastic lymphoma.32 When we applied this model to our cohort, OS and DFS were significantly improved in patients attributed to pattern A than in patients attributed to the other 2 patterns (P = .02 and P = .03, respectively). However, whereas we found remarkable parallels in overall staining frequencies for these expression markers as described by Carbone et al,32 and although the reported clustering of single markers within histologic subtypes was highly reproducible, only 53% (47 of 89) cases in our cohort could be applied to 1 of these 3 profiles. Because we were more interested in expressing profiles of prognostic value than of associations with specific pathologic categories, we used various models to include as many cases as possible. This was achieved most successfully with a model that has recently shown to be relevant in HIV-negative DLBCL.20 In this model proposed by Chang et al20 cases are classified into a GC B-cell pattern (pattern A*, expression of GC B-cell markers such as CD10 and/or BCL-6 but not of activation markers such as MUM1/IRF4 and CD138/Syn-1), an activated GC B-cell pattern (pattern B*, expression of at least one of GC B-cell markers and of one of the activation markers), and an activated non-GC B-cell pattern (pattern C*, expression of MUM1/IRF4 and/or CD138/Syn-1 but not of GC B-cell markers).20 With this model, 89% (79 of 89) of the patients in our cohort were classifiable.
Immunohistochemical expression profiles and outcomes
As shown in Figure 2E, patients with pattern C had a significantly poorer OS in the univariate analysis than those with the other 2 patterns (P = .01, when pattern A and B were both combined, P = .003). Of note, patterns A and B did not differ, suggesting that in ARL, the expression of MUM1/IRF4 or CD138/Syn-1 bears only an adverse effect when GC B-cell markers are completely lacking.
The remaining variables to be included in the Cox model for OS were CD10, CD20, and a post-GC differentiation according to Chang et al,20 a prior AIDS event, a CD4 cell count below 100/μL, a low age-adjusted IPI, and a more recent diagnosis of ARL. Four of these variables were identified as independent predictors for OS using forward selection, namely a post-GC differentiation, no prior AIDS event, a low age-adjusted IPI, and a more recent diagnosis of ARL (see Table 4). Including the use of HAART (the relative hazard of “HAART use” when compared with “no HAART use” was 0.17; 95% CI, 0.08-0.38; P < .001) instead of the year of diagnosis as a covariable in the multivariable model, the results did not change significantly (data not shown). The relative hazard for HAART use was slightly lower compared with the relative hazard of an ARL diagnosis in 1997 to 2004, but the difference was not statistically significant.
Cox models: remaining variables for overall survival and for disease-free survival
. | RH (95% CI) . | P . |
|---|---|---|
| Overall survival | ||
| No prior AIDS-defining illness, versus yes | 0.41 (0.18-0.94) | .036 |
| Diagnosis 1997-2004, versus 1989-1996 | 0.34 (0.16-0.69) | .003 |
| Post-GC differentiation, versus activated GC or GC | 3.98 (1.45-10.93) | .007 |
| Low IPI, versus high | 0.37 (0.18-0.76) | .007 |
| Disease-free survival | ||
| Post-GC differentiation, versus activated GC or GC | 35.20 (4.32-287.05) | .001 |
| Low IPI, versus high | 0.33 (0.13-0.81) | .015 |
. | RH (95% CI) . | P . |
|---|---|---|
| Overall survival | ||
| No prior AIDS-defining illness, versus yes | 0.41 (0.18-0.94) | .036 |
| Diagnosis 1997-2004, versus 1989-1996 | 0.34 (0.16-0.69) | .003 |
| Post-GC differentiation, versus activated GC or GC | 3.98 (1.45-10.93) | .007 |
| Low IPI, versus high | 0.37 (0.18-0.76) | .007 |
| Disease-free survival | ||
| Post-GC differentiation, versus activated GC or GC | 35.20 (4.32-287.05) | .001 |
| Low IPI, versus high | 0.33 (0.13-0.81) | .015 |
RH indicates relative hazards for death from any cause (overall survival) and for relapse or death from any cause (disease-free survival); GC, germinal center; IPI, International Prognostic Score.
Because there was a moderate-to-high correlation between CD10, CD20, and post-GC differentiation, these markers or profiles could not be identified as independent from each other, and only one marker or profile remained in the model. However, when controlling CD10 or CD20 expression each solely for age-adjusted IPI, prior AIDS and year of ARL diagnosis (early versus recent), they remained significantly associated with OS. OS was significantly improved in cases with CD10 expression (hazard ratio [HR], 0.47; 95% CI, 0.24-0.92; P = .03) and with CD20 expression (HR, 0.24; 95% CI, 0.10-0.60; P = .002).
We also performed Cox regression analysis for DFS. In this model, only a low IPI and a post-GC differentiation were identified as independent prognostic factors (see Table 4). These results did not change significantly when patients who died of AIDS without evidence of recurrence were censored (data not shown).
Discussion
To our knowledge, this is the first study analyzing the association between immunohistochemical expression profiles and their relation to clinical course and characteristics in a large cohort of AIDS-related lymphoma. We demonstrated that the presence of a signature indicating a post-germinal center (GC) stage is associated with a worse OS and DFS when compared with ARL with an activated GC or GC signature. Beside a prior AIDS diagnosis, an early ARL diagnosis (in the pre-HAART era) and a high IPI, a post-GC signature was independently predictive for a worse OS and a worse DFS. When the use of HAART (which was also significantly associated with a better prognosis) was included in the model instead of the year of diagnosis, results did not change significantly.
Thus, our findings implicate the clinical relevance of the histogenetic model for ARL that was proposed by Carbone et al31,32 in this journal. This model segregates 3 major phenotypic patterns, reflected by the expression of the 3 markers BCL-6, MUM1/IRF4, and CD138/Syn-1, which correspond to distinct stages of physiologic B-cell development. However, we propose the addition of CD10 to these profiles. CD10 is a cell-surface metalloproteinase that has a restricted expression in the germinal center cells and that has been shown to be a reliable marker of follicular center B-cell differentiation.45
Applying our expression results to a model that includes CD10 as a further marker,20 a significant larger group of cases could be classified in our cohort. In addition, we showed that in ARL the presence of activation markers such as MUM1/IRF4 and CD138/Syn-1 bears only an adverse effect when GC markers such as BCL-6 or CD10 are completely lacking. The differences between phenotypic patterns regarding outcome remained also significant when only patients with low or high IPI were analyzed (data not shown).
This study also showed CD10 expression (alone and as part of the GC expression profile) to be predictive for an improved OS. This was seen in the whole cohort but was also seen in the subgroup of DLBCL. It should be mentioned that CD10 expression was not predictive with regard to DFS and that CD10 was significantly associated with a preserved immunocompetence at the time of ARL diagnosis. However, CD10 expression has been shown to be predictive for OS in several studies of different cohorts of HIV-negative NHL.18-20,24,26 CD10 expression is also associated with increased apoptosis and proliferation in HIV-negative DLBCL.46 Our analysis of expression of Ki-67 protein with monoclonal antibodies41 revealed a trend toward a higher proliferation in cases expressing CD10. Given that the classification of lymphoma in patients infected with HIV is difficult even for experienced pathologists and that most studies on ARL found no association between distinct histologic features and outcome,9-15 we recommend that CD10 be included in the histopathologic routine panel of ARL diagnosis.
In addition to CD10 expression, CD20 expression also was of prognostic value concerning OS and DFS in our study. Of note, the cohort had a relatively high frequency of CD20- cases (25%), reflecting not only a relatively large subgroup of plasmablastic lymphoma but also some lymphomas that resembled morphologically and immunohistochemically primary effusion lymphoma. These entities typically lack CD20 expression, and some small studies have reported on a poor outcome of patients with these lymphomas.41,47-49 CD20 expression was associated with a higher frequency of an elevated LDH, indicating that these ARL have a higher proliferation rate. Indeed, CD20 expression was associated with a higher percentage of cells that stained positive for the proliferation marker Ki-67.
Our findings may have important implications regarding therapeutic strategies. Given the enhanced tolerability of chemotherapy in patients infected with HIV in the HAART era50-52 and given the poor outcome in patients without CD10 and/or CD20 expression or with a post-germinal profile (almost all of them were treated with CHOP-based regimens), one could consider more intensive approaches in these patients. However, our retrospective data should be confirmed by prospective studies.
As previously reported,53,54 we rarely found an expression of BCL-2 in ARL. This may be also of clinical relevance, as studies on HIV-negative DLBCL indicate that patients without BCL-2 expression may not benefit from the additional use of monoclonal antibodies such as rituximab.55,56 In the light of newer data on high rates of infectious complications in patients with ARL treated with rituximab in one large randomized trial,57 the use of this monoclonal antibody remains questionable in patients infected with HIV. In our cohort, only one patient received rituximab. Of note, BCL-2 had no effect on DFS and OS in our cohort, regardless of which subgroup was analyzed. This contrasts with many studies in the HIV-negative setting.19,21,23,25,27-29 The reason for this difference remains unclear. However, the low expression of BCL-2 in ARL suggests that this protooncogene is infrequently involved in the pathogenic mechanisms contributing to ARL.
We were also interested in the changes of the expression profiles of ARL over time, because there is conflicting data on changes in the pathologic spectrum of ARL in the era of HAART. Whereas some studies revealed no significant changes,58,59 other investigators found larger proportions of cases with an unknown histology or a histology other than DLBCL or BL.2 In contrast, some reported on an increase of DLBCL60 in the HAART era. Data on changes in expression profiles over time are lacking. However, some investigators hypothesized that the improved immune function associated with HAART may lead to a shift in pathogenesis away from lymphomas of post-germinal center B-like origin.53 Such a shift was not evident when we performed a correlational analysis determining the influence of HAART by defining different time intervals according to changes in the availability of antiretroviral therapy. However, when analyzing possible HAART effects on the individual patient data level, the expression of CD20 was rarely seen in patients who were treated with HAART before ARL occurred, although the numbers of such patients in our cohort were too low to draw definite conclusions. Moreover, the time period on HAART at ARL diagnosis was relatively short in some patients, indicating that at least in some individuals the events triggering lymphogenesis had possibly already occurred at the time of HAART initiation. Future studies should analyze possible HAART effects on the individual patient data level because many ARL occur in untreated patients. In our previous study on 203 cases, only 46% of the patients developing ARL between 1997 and 2001 had received HAART at the time of ARL diagnosis.10
In summary, our data demonstrate the clinical relevance of immunohistochemical expression profiles that correspond to distinct stages of physiologic B-cell development. They also indicate that CD10, alone or as part of the post-GC expression profile, and CD20 expression are important predictors of clinical outcome in AIDS-related lymphoma. Given the poor outcome of patients with ARL, indicating a post-germinal center signature, these observations may, if confirmed by prospective studies, lead to distinct therapeutic strategies.
Prepublished online as Blood First Edition Paper, May 19, 2005; DOI 10.1182/blood-2004-12-4631.
C.H. and M.T. contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal