Abstract
Dendritic cells (DCs) have the unique ability to initiate primary immune responses, and they can be conditioned for vaccinal purposes to present antigens after the engulfment of apoptotic cells. To recruit the rare antigen-specific naive T cells, DCs require a maturation step and subsequent transport toward lymph node (LN). To date, prostaglandin E2 (PGE2) is the best-characterized compound inducing this LN-directed migration in vitro, but PGE2 may skew the immune responses in a TH2 direction. We demonstrate here that on incubation with apoptotic tumor cells and tumor necrosis factor-α (TNF-α) or lipopolysaccharide (LPS), human monocyte-derived DCs become fully mature and acquire high migratory capacities toward LN-directing chemokines. The migration of TNF-α-treated DCs occurs only after cotreatment with apoptotic cells but not with necrotic cells. DC migration requires CD36 expression and incubation with apoptotic cells in the presence of heat-labile serum components. Moreover, on treatment with apoptotic cells and LPS, the migrating DCs are able to recruit naive T cells to generate TH1 immune responses. Our results show that the cotreatment of DCs with apoptotic tumor cells and inflammatory signals is promising for the design of an antitumoral DC-based vaccine. (Blood. 2005;106:1734-1741)
Introduction
Myeloid dendritic cells (DCs) have the unique ability to activate naive T cells and, as such, can be tailored to initiate an immune response against neoantigens.1 Once loaded with antigens in peripheral tissues, DCs must migrate to secondary lymphoid organs and reach a fully mature functional stage to recruit rare naive T cells.2 In the past decade, in vitro-generated DCs have been involved as cell adjuvants in multiple tumor vaccination trials to trigger antitumor immune responses and tumor regression.3 For tumor vaccination purposes, autologous DCs are generated either from CD34+ progenitors4 or from peripheral blood monocytes differentiated in the presence of granulocyte macrophage-colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4)5 or IL-13.6,7 DCs are then loaded with tumor antigens, matured, and reinjected into patients. Different protocols have been proposed to load DCs with tumor antigens; the most common forms of antigen are preprocessed peptides, tumor cell lysates, whole apoptotic cells, and necrotic tumor cells (for a review, see Schuler et al8 ). Numerous in vivo and in vitro assays have been used to compare the capacities of DCs loaded with the different forms of antigen to activate naive T cells.9-14 Antigen loading using whole apoptotic or necrotic tumoral cells and cell lysates allows the presentation of an entire set of tumor antigens by major histocompatibility complex (MHC) class I and class II molecules,15-17 which seems advantageous for recruiting large CD4 and CD8 T-cell repertoires.18 Moreover, the activation of CD4 T cells appears essential because it has been shown that CD8 activation without CD4 help may tolerize CD8 T cells19 and preclude the establishment of CD8 memory T cells.20 Indeed, the CD40/CD40L signalization pathway, probably engaged by CD4 cells, is needed to induce full DC maturation and effective activation of CD8 T cells.21 Immature DCs can internalize apoptotic bodies through multiple mechanisms, among which the recognition of altered self by CD36,22 integrins αvβ3 and αvβ5,23 phosphatidylserine receptor, and the molecular complex iC3b/C1q/Calreticulin/CD9124 play crucial roles (for a review, see Savill et al25 ). These various receptors can mediate intracellular signaling events, thereby modifying DC responses to inflammation stimuli and influencing their migration properties24 and their antigen-presentation capacity.26
It is generally accepted that reinjected DCs loaded with tumor antigens must have the capacity to migrate to lymph nodes (LNs), encounter naive T cells, and activate them toward a TH1 phenotype8,18 to induce efficient antitumoral cytotoxic responses. This dual ability of DCs to migrate toward secondary lymphoid organs and to orient the immune response is, in our opinion, an important criterion for the design of DC-based vaccines. DC maturation agents such as tumor necrosis factor-α (TNF-α), lipopolysaccharide (LPS), and CD40L have been used to confer these functional attributes to DC preparations.27 Among the numerous phenotypic and functional changes, DC maturation signals down-regulate internalization capacities and trigger the expression of the chemokine receptor CCR7, allowing the migration of mature DCs toward the LN-directing chemokines CCL19/MIP3β and CCL21/6Ckine.28 Recent results have highlighted that, in addition to CCR7, the proinflammatory agent prostaglandin E2 (PGE2) is essential for promoting the migration of mature DCs toward LN chemokines in vitro.29,30 PGE2 tends, however, to produce DCs that skew the immune responses toward a TH2 orientation,31 impairing the use of PGE2 as an inductor of DC migration in antitumoral clinical trials. A recent report32 indicates that apoptotic cell (ApoC) interaction favors the migration of a small fraction of DCs toward LN chemokines in the absence of additional maturation stimuli. We think it is essential to further define the ApoC material and the inflammatory stimuli required for efficient induction of DC migration. From a clinical perspective, it is equally important to assess the induction of TH1 responses using DCs that have migrated toward LN chemokines.
We report here that the preincubation of immature DCs with ApoC in the presence of TNF-α or LPS allowed efficient migration of mature DCs toward LN chemokines, comparable to the migration induced by PGE2. Productive ApoC/DC interaction was independent of PGE2 release but required a heat-sensitive complement fraction and CD36. Finally, we found that ApoC interaction and LPS maturation signal empower migrating DCs to recruit alloreactive naive T cells and induce TH1 responses.
Materials and methods
Reagents and antibodies
Culture media included RPMI (Invitrogen, Cergy Pontoise, France) supplemented with 2 mM L-glutamine and 10% fetal bovine serum (FBS) for cell lines and AIMV (Invitrogen) supplemented with 500 U/mL GM-CSF and 50 ng/mL IL-13 for the DC differentiation culture, referred to as the DC medium. DC medium was supplemented with 10% autologous serum for migration experiments or 10% human AB serum for mixed-leukocyte reaction (MLR) experiments. GM-CSF (Leucomax) was obtained from Schering-Plough (Levallois-Perret, France), IL-13 was obtained from Sanofi-Synthélabo (Paris, France), TNF-α and chemokines CCL19 (MIP-3β/Exodus-3) and CCL21 (6Ckine/Exodus-2) were provided by AbCys (Paris, France), interferon-γ (IFN-γ; Imukin) was obtained from Boehringer (Meylan, France), PGE2 (Prostine E2) was obtained from Pharmacia/Pfizer (Amboise, France), and LPS was obtained from Sigma (St Quentin Fallavier, France). Antibodies for cell culture, anti-Fas (clone CH11), anti-CD36 (clone FA6-152), and anti-CD51 (clone 69.6.5) were provided by Beckmann-Coulter (Roissy, France). Antibodies for fluorescence-activated cell sorter (FACS) analysis and confocal microscopy, anti-CD83 fluorescein isothiocyanate (FITC), anti-CD11c-PC5 and anti-CD11c-allophycocyanin (anti-CD11c-APC), anti-IFN-γ-FITC and anti-IL-4-phycoerythrin (anti-IL-4-PE), anti-CCR7, biotinylated anti-mouse antibody, PE-coupled avidin, Annexin-V-PE, and 7AAD were obtained from Becton Dickinson (Le Pont de Claix, France). Anti-CD3-PC5 and anti-CD1a-PC5 antibodies were from Beckmann-Coulter. Intracellular and nuclear stain reagents chloromethyl-benzoylaminotetramethylrhodamine (CMTMR), carboxyfluorescein diacetate succinimidyl ester (CFSE), and 4,6-diamidino-2-phenylindole-2-HCl (DAPI) were purchased from Molecular Probes/Interchim (Montluçon, France).
Cell cultures
Jurkat, a T-cell line isolated from a patient with acute myeloid leukemia (AML), was kindly donated by L. Amiot (Laboratoire d'Hématologie, Rennes, France). M44, a melanoma-derived cell line, was a kind gift from F. Jotereau (INSERM U463, Nantes, France). U251, a glioblastoma cell line, was a kind gift from V. Catros-Quemener (UPRESA CNRS 6027, Rennes, France). Buffy coats and autologous sera were collected from EFS Bretagne (Rennes, France). Immature monocyte-derived DCs were routinely generated from the CD14+-selected fraction of peripheral blood mononuclear cells (PBMCs). PBMCs isolated using the Ficoll-Methrizoate technique were purified with anti-CD14 magnetic beads, according to the manufacturer's instructions (Miltenyi Biotec, Paris, France). Alternatively, monocytes were purified from PBMCs by negative selection using anti-CD2, anti-CD3, anti-CD19, anti-CD56, anti-CD66b, and anti-glycophorin A magnetic beads (StemCell Technologies, Meylan, France). In both cases, pure monocytes (routinely more than 80% CD14/CD11c/HLA-DR triple-positive cells) were plated in DC differentiation medium at 1 × 106 cells/mL in culture bags (Stedim, Aubagne, France). Half the media were replaced on day 2 or 3, and immature DCs were harvested on day 6.
Apoptosis induction of cell lines and interaction with DCs
Various apoptotic stimuli have been tested on Jurkat, M44, and U251 cell lines. A 40-J/cm2 or an 80-J/cm2 ultraviolet B (UVB) stimulus was chosen for Jurkat and M44/U251 cells, respectively, rather than serum deprivation or Fas-mediated cell death. In our hands, serum deprivation induced low levels of cell death. Jurkat cells were sensitive to Fas-induced cell death, but M44 and U251 cells were not. Moreover, anti-Fas antibody (clone CH11) would have added bias to the internalization process of apoptotic bodies because of the presence of Fc-receptors on immature DCs.33 UVB irradiation had 2 main advantages: it did not add chemicals or antibodies to the culture medium, and it induced early, massive apoptosis of the cells tested (data not shown).
In some experiments, Jurkat cells were stained by incubation for 30 minutes, at 37°C, with 5 μM CMTMR (Molecular Probes). Stained or unstained Jurkat cells were plated at 1 × 106 cells/mL in AIMV in 6-well plates and were induced to apoptosis by UVB irradiation (40 J/cm2) using a UV solar simulator (Müller Elektronik, Salzkotten, Germany). Under these conditions, more than 60% of the cells were Annexin-V positive 2 hours after irradiation; 24 hours later, 100% of the cells fixed 7AAD. Irradiated Jurkat cells were then washed, resuspended in AIMV medium, and added to immature DCs to obtain the indicated DC/ApoC ratio. Immature DCs, supplemented or not supplemented with ApoC, were plated at 5 × 105 cells/mL in DC medium plus 10% complete autologous serum. When indicated, serum was heat inactivated for 30 minutes at 56°C. ApoCs and DCs were then allowed to interact for 4 hours before the addition of 250 U/mL TNF-α, 5 μg/mL LPS, or 1 μg/mL PGE2. After 48-hour incubation, DCs were harvested and counted.
DC phenotyping
For each staining, 100 000 DCs were incubated for 30 minutes with fluorescence-labeled antibodies, in the presence of phosphate-buffered saline (PBS) and 10% AB serum, to block unspecific staining. For CCR7 detection, because of the weak expression of this antigen, we used a staining process consisting of 3 steps—unstained anti-CCR7 or isotype control antibody, anti-mouse biotinylated antibody, and PE-coupled avidin. Stained cells were analyzed using FACScan (Becton Dickinson). In some experiments, stained cells (DAPI cells, CMTMR-stained ApoCs, and CD11c-APCs) were deposed on slides by centrifugation, fixed in 2% paraformaldehyde, and mounted in Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL), for analysis on a Leica TCS SP2 confocal microscope (Leica, Mannheim, Germany), using oil immersion 40 × 1.25 NA and 100 × 1.4 NA objectives and Leica confocal software. Images were processed using Adobe Photoshop 7.0.1 (Adobe Systems, San Jose, CA).
Characterization of the migratory properties of DCs
DCs were suspended in AIMV media plus 10% AB serum at 1 × 106 DC/mL. To assess the migratory capacities of DCs, we used a 12 well-Transwell microplate (Corning, Amboise, France) with a 5-μm membrane pore size that forbade the passive diffusion, but allowed the active migration, of DCs. For each condition tested, lower chambers of the Transwell (Corning) were filled with 600 μL AIMV plus nothing or plus 300 ng/mL CCL19 or 250 ng/mL CCL21. DCs (1 × 105) were deposited in the upper chamber of the Transwell (Corning) and were allowed to migrate for 3 hours at 37°C. Migrating DCs were harvested from the lower chamber and were counted for 60 seconds using FACS.29 In some experiments, enumerations were performed twice to assess the reliability of the method. We never found more than 10% variation between 2 counts.
Dosage of PGE2
After 2 days of incubation, DCs were spun down, and the supernatants were kept at -80°C. The PGE2 content of supernatants was measured using a competitive enzyme-linked immunosorbent assay (ELISA) kit (R&D, Lille, France) according to the manufacturer's instructions.
MLR
Samples of total DCs collected before deposit in the upper chamber or migrating DCs collected in the lower chamber were plated in 96-well microplates. Because of the low number of migrating DCs, only one APC/T-cell ratio was feasible. After preliminary experiments, half-maximal T-cell stimulation was reproducibly obtained at a 1:40 APC/T-cell ratio. We thus added 100 000 allogeneic CFSE-stained CD3+ T cells from CD14- fractions to 2500 DCs. APC/T-cell mixtures were cultured in AIMV medium plus 10% human AB serum for 3 to 4 days before CD3-PC5 staining and FACS analysis. The decrease in the level of CFSE staining was used to assess T-cell division in response to DCs.
Determination of the percentage of IFN-γ-producing T cells
Total DCs or migrating DCs were counted, and 2500 DCs were plated in each well of 96-well microplates. One hundred thousand unstained allogeneic CD4+/CD45RA+ T cells, purified using the naive human stem cell CD4+ T-cell enrichment kit (greater than 95% CD4+/CD45RA+ cells), were added to the DCs and incubated in X-VIVO medium (Cambrex, Verviers, Belgium) for 6 days. After expansion in the presence of IL-2 (50 U/mL) for another 6 days, cells were stimulated with 50 ng/mL phorbol myristate acetate (PMA; Sigma-Aldrich) and 500 ng/mL ionomycin (Sigma-Aldrich) for 4 hours. Golgi-Stop (Becton Dickinson) was added for the last 2 hours. Cells were then intracellularly stained for IFN-γ and IL-4 using the intracellular cytokine staining kit (Becton Dickinson), according to the manufacturer's instructions.
Results
DC migration after ApoC interaction and TNF-α-induced maturation
In preliminary experiments, we found that maximal DC migration toward CCL21 was readily achieved after 48-hour incubation with the reference PGE2/TNF-α treatment, as reported previously.29,30 To explore here the most favorable conditions needed to induce DC migration after ApoC treatment, we assessed the migration capacities and CCR7/CD83 coexpression of human DCs at this 48-hour point.
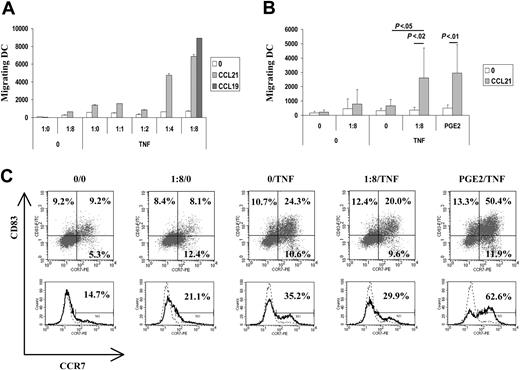
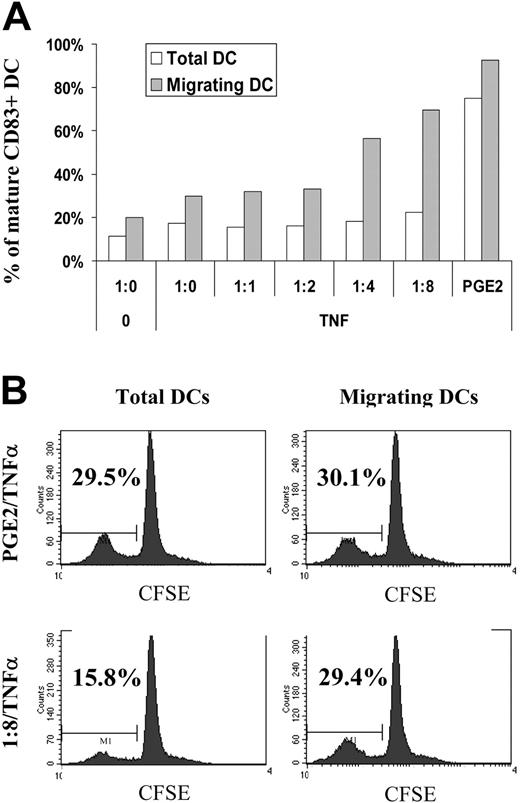
DCs treated with ApoC alone or with TNF-α alone exhibited moderate activation of migration capacity toward CCL21 compared with unstimulated DCs (Figure 1A-B). However, treatments with ApoC and TNF-α were strongly synergistic, promoting the migration capacity of DCs in an ApoC dose-dependent manner (Figure 1A) and reaching a plateau at a DC/ApoC ratio of 1:8 (1:8 TNF-α). As determined in more than 5 different experiments, the 1:8 ratio of TNF-α DCs that migrated toward CCL21 were as numerous as those that migrated after maturation, driven by the combination of TNF-α and PGE2. Under these 2 optimum conditions, DC migration was 35 to 40 times higher than that of unstimulated DCs and 6 to 8 times higher than that of DCs stimulated with ApoC or TNF-α alone. Migration of the 1:8 TNF-α-treated DCs toward CCL21 was significantly different (P < .02) from nonspecific migration (Figure 1B). Moreover, migration toward CCL21 of 1:8 TNF-α DCs was significantly different (P < .05) from migration toward CCL21 of DCs treated with TNF-α alone. Similar results were obtained using the CCL19 chemokine (Figure 1A) or using DCs differentiated from monocytes purified by negative selection (data not shown). DC phenotyping showed that ApoC alone did not increase the expression of the maturation marker CD83 but slightly increased the expression of CCR7 on immature DCs (Figure 1C). Furthermore, we found that TNF-α alone induced CD83 and CCR7 coexpression to levels equivalent to those observed with the combination of ApoC and TNF-α (Figure 1C). Finally, PGE2 plus TNF-α dramatically increased CD83 and CCR7 expression (Figure 1C). Thus, the induction of DC migration with ApoC treatment is not correlated with overall CD83 and CCR7 surface expression levels.
Apoptotic cells induce migration of mature DCs toward the LN chemokines CCL19 and CCL21. Immature DCs were exposed, in 10% autologous complete serum, to apoptotic Jurkat cells for 4 hours before the addition of TNF-α (250 U/mL). Migration capacity of DCs toward CCL19 (300 ng/mL) or CCL21 (250 ng/mL) was tested 2 days later using the Transwell migration assay. (A) Migration of DCs alone (1:0/0) plus TNF-α (1:0/TNF) or treated with apoptotic cells at different DC/ApoC ratios (1:1, 1:2, 1:4, 1:8). Error bars represent the SD of 2 counts. (B) Mean ± SD of 5 to 8 independent experiments. Statistical analyses were performed using the Student t test. PGE2 (1 μg/mL) was added simultaneously with TNF-α. Unless otherwise stated, differences were not significant. The migration difference between no attractant and CCL21 was significant for the condition PGE2/TNF-α (P < .01) and for the condition 1:8/TNF (P < .02). The difference between 1:8/TNF with CCL21 and 0/TNF with CCL21 was significant (P < .05). (C) CD83 and CCR7 co-expression (top) or CCR7 expression (bottom) after 48-hour incubation in various conditions. Experimental conditions and DC/ApoC ratios are similar to those in panels A and B. Histograms are representative of more than 7 experiments. (Top) Percentages of cells in each quadrant are given. (Bottom) Percentages of CCR7-positive cells as defined using isotype control (dashed line) are given.
Apoptotic cells induce migration of mature DCs toward the LN chemokines CCL19 and CCL21. Immature DCs were exposed, in 10% autologous complete serum, to apoptotic Jurkat cells for 4 hours before the addition of TNF-α (250 U/mL). Migration capacity of DCs toward CCL19 (300 ng/mL) or CCL21 (250 ng/mL) was tested 2 days later using the Transwell migration assay. (A) Migration of DCs alone (1:0/0) plus TNF-α (1:0/TNF) or treated with apoptotic cells at different DC/ApoC ratios (1:1, 1:2, 1:4, 1:8). Error bars represent the SD of 2 counts. (B) Mean ± SD of 5 to 8 independent experiments. Statistical analyses were performed using the Student t test. PGE2 (1 μg/mL) was added simultaneously with TNF-α. Unless otherwise stated, differences were not significant. The migration difference between no attractant and CCL21 was significant for the condition PGE2/TNF-α (P < .01) and for the condition 1:8/TNF (P < .02). The difference between 1:8/TNF with CCL21 and 0/TNF with CCL21 was significant (P < .05). (C) CD83 and CCR7 co-expression (top) or CCR7 expression (bottom) after 48-hour incubation in various conditions. Experimental conditions and DC/ApoC ratios are similar to those in panels A and B. Histograms are representative of more than 7 experiments. (Top) Percentages of cells in each quadrant are given. (Bottom) Percentages of CCR7-positive cells as defined using isotype control (dashed line) are given.
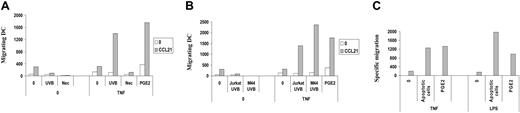
Next we sought to determine whether the action of ApoC on DC migration was attributed to early ApoC or to late apoptotic/necrotic cells. We used 30-minute incubation at 55°C as a necrosis-inducing stimulus.34 Necrotic cells had no effect on DC migration under these conditions, in contrast to early ApoC (Figure 2A). Moreover, the induction of DC migration to LN chemoattractants by ApoC was not limited to Jurkat cells because apoptotic melanoma cell line M44 (Figure 2B) and glioblastoma cell line U251 (data not shown) induced an even higher level of DC migration than apoptotic Jurkat cells.
DC migration toward CCL21 occurs with apoptotic and not with necrotic cells and does not depend on the cell line or on the maturation stimulus used. (A) Immature DCs were exposed for 4 hours to UVB-treated ApoC (UVB) or to necrotic (Nec) Jurkat cells at a DC/dead cell ratio of 1:8 before the addition of TNF-α (250 U/mL). (B) DCs were exposed to apoptotic Jurkat cells (Jurkat UVB) or M44 cells (M44 UVB) before the addition of TNF-α (250 U/mL). (C) DCs were stimulated with apoptotic Jurkat cells (apoptotic cells) before the addition of TNF-α (250 U/mL) or LPS (5 μg/mL). Specific migrating DC numbers represent the numbers of DCs migrating toward CCL21 minus the numbers of DCs migrating toward medium. Nonspecific migration accounted for 342 ± 180 DCs in the TNF-α condition and 1618 ± 834 DCs in the LPS condition compared with 421 ± 403 DCs in the absence of treatment (not shown).
DC migration toward CCL21 occurs with apoptotic and not with necrotic cells and does not depend on the cell line or on the maturation stimulus used. (A) Immature DCs were exposed for 4 hours to UVB-treated ApoC (UVB) or to necrotic (Nec) Jurkat cells at a DC/dead cell ratio of 1:8 before the addition of TNF-α (250 U/mL). (B) DCs were exposed to apoptotic Jurkat cells (Jurkat UVB) or M44 cells (M44 UVB) before the addition of TNF-α (250 U/mL). (C) DCs were stimulated with apoptotic Jurkat cells (apoptotic cells) before the addition of TNF-α (250 U/mL) or LPS (5 μg/mL). Specific migrating DC numbers represent the numbers of DCs migrating toward CCL21 minus the numbers of DCs migrating toward medium. Nonspecific migration accounted for 342 ± 180 DCs in the TNF-α condition and 1618 ± 834 DCs in the LPS condition compared with 421 ± 403 DCs in the absence of treatment (not shown).
Finally, we sought to determine whether the effect of ApoC on DC migration was restricted to DCs that matured through TNF-α by considering the role of LPS as an independent maturation stimulus acting through the TLR4 pathways. We found that LPS increased ApoC-induced migration in a manner equivalent to that for TNF-α (Figure 2C), indicating that different activator pathways have a similar effect on the acquisition of migratory properties in ApoC-pulsed DCs.
Synergy between ApoC interaction, TNF-α, and PGE2
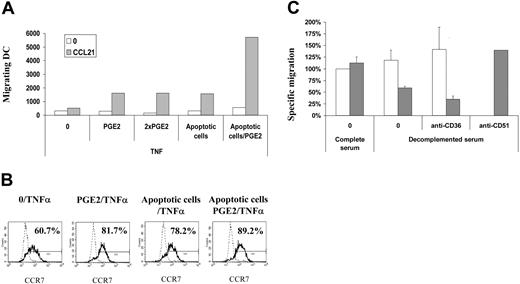
To evaluate the possibility that ApoC-DC interaction triggers PGE2 release, either by DCs in a paracrine manner or by the cells undergoing apoptosis, we measured PGE2 content in ApoC-DC cultures and analyzed the synergy between ApoC, TNF-α, and PGE2 on DC migration. PGE2 levels were below the detection limit of 16 pg/mL in the DC supernatant after 48-hour DC/ApoC interaction, with or without TNF-α or LPS. Thus, ApoC did not trigger PGE2 release by DCs. To confirm the lack of implication of PGE2 in ApoC-mediated DC migration, we studied the additive effect of PGE2 and ApoC on DC migration. As previously observed, the combinations PGE2/TNF-α and ApoC/TNF-α had similar effects on DC migration (Figure 3A). However, treatment with ApoC/PGE2/TNF-α had a dramatic synergistic effect, increasing DC migration 5- to 10-fold compared with ApoC/TNF-α and PGE2/TNF-α stimuli (Figure 3A). In contrast, increasing the PGE2 concentration 2-fold did not increase DC migration. These results are exemplified by absolute numbers of cells that migrated. In the PGE2/TNF-α condition, a deposit of 100 000 DCs (total DCs) in the Transwell (Corning) upper chamber resulted in the migration of 18 700 DCs (counted in the lower chamber, migrating DCs). This 18.7% migration was comparable to the 18.2% migration obtained in the ApoC/TNF-α condition. In the ApoC/PGE2/TNF-α condition, however, migrating DCs represented 66% of total DCs. These data strongly suggest that ApoC and PGE2 stimuli act through independent mechanisms.
To relate these functional results with DC phenotype, we measured the expression level of CCR7 on DCs under the same experimental conditions and found that TNF-α induced the expression of CCR7 on 60% of the cells (Figure 3B). Adding PGE2 to TNF-α at a single or a double concentration and adding ApoC resulted in a proportion of approximately 80% CCR7 expression. The TNF-α/PGE2/ApoC combination, which induced a dramatic increase in DC migration, only slightly elevated the percentage of CCR7+ cells without increasing the mean fluorescence intensity in these DC populations. These experiments confirm that the level of CCR7 expression is loosely correlated with the ability of DCs to migrate in response to CCL21.
Apoptotic cells act independently of PGE2 and CD51 on DC migration but require complement and CD36. DCs were exposed to apoptotic Jurkat cells 4 hours before incubation with TNF-α (250 U/mL) or PGE2 (3.5 μg/mL and 7.0 μg/mL for 1 × PGE2 and 2 × PGE2, respectively) for 2 days. (A) Migration capacities of DCs tested using the Transwell migration assay. (B) Surface expression of CCR7 examined in total DC fractions by flow cytometry. Percentages of CCR7-positive cells as defined using isotype control (dashed line) are given. (C) DCs were incubated in 10% autologous complete or heat-inactivated serum, in the presence of 10 μg/mL blocking anti-CD51 or anti-CD36 antibodies for 30 minutes before the addition of PGE2 (□) or apoptotic Jurkat cells (▦). For clarity, specific migrations, normalized with the PGE2/TNFα/complete serum condition, are depicted (mean ± SD of 3 experiments). As in Figure 2C, specific migration represents the number of DCs migrating toward CCL21 minus the number of DCs migrating toward medium.
Apoptotic cells act independently of PGE2 and CD51 on DC migration but require complement and CD36. DCs were exposed to apoptotic Jurkat cells 4 hours before incubation with TNF-α (250 U/mL) or PGE2 (3.5 μg/mL and 7.0 μg/mL for 1 × PGE2 and 2 × PGE2, respectively) for 2 days. (A) Migration capacities of DCs tested using the Transwell migration assay. (B) Surface expression of CCR7 examined in total DC fractions by flow cytometry. Percentages of CCR7-positive cells as defined using isotype control (dashed line) are given. (C) DCs were incubated in 10% autologous complete or heat-inactivated serum, in the presence of 10 μg/mL blocking anti-CD51 or anti-CD36 antibodies for 30 minutes before the addition of PGE2 (□) or apoptotic Jurkat cells (▦). For clarity, specific migrations, normalized with the PGE2/TNFα/complete serum condition, are depicted (mean ± SD of 3 experiments). As in Figure 2C, specific migration represents the number of DCs migrating toward CCL21 minus the number of DCs migrating toward medium.
Mechanisms of DC/ApoC interactions
To further analyze the implications of molecular components involved during the tethering of ApoC on immature DCs, we assessed the roles of (1) the C1q/calreticulin/CD91 complex, which recognizes iC3b coated on the ApoC surface,24 by inactivating iC3b, which is the thermosensitive component of the complement, (2) the integrin αv-chain (CD51), which, coupled to the integrin β5 or the integrin β3-chain, can recognize ApoC with the help of TSP1 and CD36,23 and (3) the CD36 receptor, which can recognize independently oxidized lipoproteins/phosphatidylserine at the ApoC surface.22
DCs incubated with heat-inactivated autologous serum (56°C for 30 minutes) showed a 50% down-regulation in migration toward LN chemokines (Figure 3C). Anti-CD36 antibodies were used only with heat-inactivated serum to avoid complement-mediated lysis of DCs. This latter treatment again decreased migration by 40% compared with migration in heat-inactivated serum alone. The association of heat-inactivated serum and anti-CD36 resulted in more than 70% inhibition of the ApoC-mediated migration. Neither heat-inactivated serum nor anti-CD36 antibodies had an effect on PGE2-mediated migration alone. Finally, anti-CD51 antibodies had no effect (Figure 3C) on the ApoC-mediated migration of DCs. Similar results were obtained using the CCL19 chemokine as a chemoattractant (data not shown). Thus, the increase in migration capacities of DCs on ApoC challenge is partly mediated through a thermosensitive component of the serum, likely iC3b, and the CD36 receptor.
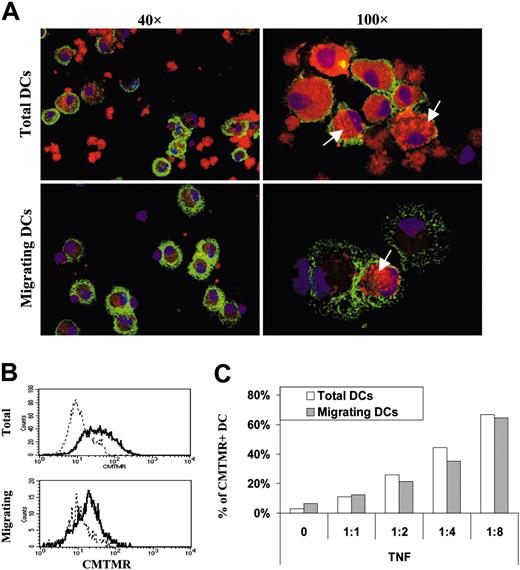
DC interaction with apoptotic cell fragments. DCs were exposed to different amounts of apoptotic Jurkat cells stained with CMTMR. DCs having internalized apoptotic cells (CMTMR+ DC) were detected by confocal or FACS analysis, before (total DC) or after (migrating DC) migration in response to CCL21. (A) Confocal pictures of total or migrating ApoC-loaded DCs at a 1:4 ratio. DCs loaded with CMTMR-labeled apoptotic cells (red) were stained with CD11-APC (green) and DAPI (blue), deposed on a microscope slide, and fixed in 2% paraformaldehyde (PFA). Arrows point to discrete intracellular accumulations of CMTMR-labeled apoptotic cell fragments in the cytosol of DCs. Fields represent 175 × 250 μm and 70 × 100 μm for 40 × and 100 × objective magnifications, respectively. (B) Representative FACS histograms under conditions similar to those in panel A. Solid and dashed lines represent fluorescence of DCs incubated with or without CMTMR-stained apoptotic cells, respectively. (C) Percentages of CMTMR+ DCs (total and migrating) as a function of the DC/ApoC ratio were determined from FACS profiles in panel B, above a CMTMR fluorescence intensity of 2 × 10.1
DC interaction with apoptotic cell fragments. DCs were exposed to different amounts of apoptotic Jurkat cells stained with CMTMR. DCs having internalized apoptotic cells (CMTMR+ DC) were detected by confocal or FACS analysis, before (total DC) or after (migrating DC) migration in response to CCL21. (A) Confocal pictures of total or migrating ApoC-loaded DCs at a 1:4 ratio. DCs loaded with CMTMR-labeled apoptotic cells (red) were stained with CD11-APC (green) and DAPI (blue), deposed on a microscope slide, and fixed in 2% paraformaldehyde (PFA). Arrows point to discrete intracellular accumulations of CMTMR-labeled apoptotic cell fragments in the cytosol of DCs. Fields represent 175 × 250 μm and 70 × 100 μm for 40 × and 100 × objective magnifications, respectively. (B) Representative FACS histograms under conditions similar to those in panel A. Solid and dashed lines represent fluorescence of DCs incubated with or without CMTMR-stained apoptotic cells, respectively. (C) Percentages of CMTMR+ DCs (total and migrating) as a function of the DC/ApoC ratio were determined from FACS profiles in panel B, above a CMTMR fluorescence intensity of 2 × 10.1
Next, to assess the effective engulfment of apoptotic cells by DCs, we incubated DCs with CMTMR-stained apoptotic cells at a 1:4 ratio and monitored by confocal microscopy the appearance of DCs containing CMTMR+ apoptotic debris in total and migratory fractions (Figure 4A). The total DC fraction contained internalized and noninternalized apoptotic cells, whereas the fraction of migrating DCs appeared devoid of free apoptotic cells. Moreover, total DCs and migrating DCs contained vesicles loaded with CMTMR+ apoptotic fragments (Figure 4A).
We then assessed that we could detect these phagocytic events using FACS analysis (Figure 4B) and monitored the percentage of apoptotic cell-loaded DCs before and after DC migration toward CCL21 to determine whether ApoC-pulsed DCs would be enriched in the migrating fraction. Surprisingly, we observed no such enrichment after DC migration, in contrast to the total DC fraction before migration (Figure 4C), providing an indication that ApoC internalization is not mandatory for DC migration.
Migrating DCs after ApoC/TNF-α or PGE2/TNF-α stimuli are enriched in mature cells. DCs were exposed to apoptotic Jurkat cells at an increasing DC/ApoC ratio before the addition of TNF-α. (A) The percentage of mature DCs was measured by CD83 expression before (total DCs) or after (migrating DCs) migration toward CCL21. (B) Total or migrating DCs were mixed at a 1:40 ratio with allogeneic T cells intracellularly stained with CFSE. At day 3, T cells were analyzed using flow cytometry. Percentages represent the fraction of proliferating cells in which CFSE was diluted at least 2-fold after 3 days.
Migrating DCs after ApoC/TNF-α or PGE2/TNF-α stimuli are enriched in mature cells. DCs were exposed to apoptotic Jurkat cells at an increasing DC/ApoC ratio before the addition of TNF-α. (A) The percentage of mature DCs was measured by CD83 expression before (total DCs) or after (migrating DCs) migration toward CCL21. (B) Total or migrating DCs were mixed at a 1:40 ratio with allogeneic T cells intracellularly stained with CFSE. At day 3, T cells were analyzed using flow cytometry. Percentages represent the fraction of proliferating cells in which CFSE was diluted at least 2-fold after 3 days.
Phenotype and antigen presentation functions of migrating DCs
It has been reported that ApoC could inhibit DC maturation35 and that immature CD83- DCs expressing the Langerin marker, likely derived from epidermal Langerhans cells, were present in LNs draining chronically inflammatory skin lesions.36 These reports raised the possibility that the migrating DCs we obtained after ApoC and TNF-α treatment were immature. To assess the state of maturation of our migrating DCs, we measured their CD83 expression and their aptitude to induce MLR. After treatment with ApoC/TNF-α or PGE2/TNF-α, migrating DCs were highly enriched in CD83+ cells (Figure 5A). It might be argued that migrating DCs are induced to mature during Transwell (Corning) migration assay because of CCR7 stimulation through CCL21 or of migration throughout the filter. CCL21 does not induce maturation of DCs; we detected no maturation of DCs on incubation in the presence of CCL21 (data not shown). We cannot exclude that migration through the filter membrane induced some level of DC maturation; however, if this were the case, we would have observed similar percentages of mature DCs for all the conditions tested. Instead, we observed that specific migration toward CCL21 (Figure 1A) and CD83 expression on migrating DCs (Figure 5A) varied in a highly correlated manner (R2 = 0.97). To perform an MLR and considering the low recovery of DCs after migration, we had to use a single, migrating DC/naive T-cell ratio. We had previously determined that a 1:40 ratio resulted in half-maximal T-cell activation (data not shown). Using this MLR assay, we observed a high correspondence between T-cell proliferation and CD83 expression. Before migration, DCs treated with ApoC/TNF-α (1:8/TNF-α) had low antigen-presenting capacity compared with the PGE2/TNF-α condition. When selecting DCs that migrated toward CCL21 after ApoC/TNF-α treatment, we observed higher levels of T-cell proliferation (Figure 5B), in agreement with a selection in the migrating DC pool of immunocompetent DCs. These data show that on ApoC plus TNF-α stimulus, migrating DCs are functionally mature. Because PGE2/TNF-α treatment induces very high levels of CD83+ cells before migration, no such enrichment of functionally mature DCs was observed after migration (Figure 5B).
Polarization of naive T cells toward a TH1 response with migrating DCs
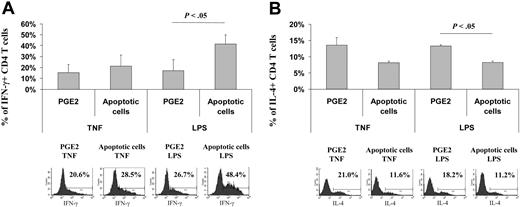
Ample evidence indicates that naive T cells must differentiate toward a TH1 phenotype to mount an efficient antitumoral response,18 leading us to test here the induction of TH1 responses with migrating DCs. DCs were matured for 48 hours in the presence of TNF-α or LPS, a well-known TH1-maturing stimulus,37 plus medium, ApoC, or PGE2. The migration patterns of these DCs toward CCL21 are shown in Figure 2C. Allogeneic naive CD45RA CD4 T cells were stimulated using DCs sorted by migration toward CCL21, but, because of the low number of migrating DCs after TNF-α or LPS stimulation alone, no data were available for such conditions. T-cell proliferation/survival rates were similar for all the conditions tested, as checked by T-cell counts. As expected, DCs induced to migrate after maturation with TNF-α and PGE2 generated a weak TH1 response (13.4% ± 4.9% of IFN-γ-positive cells) (Figure 6A). Substitution of PGE2 by ApoC increased this response only marginally (16.1% ± 8.6% of IFN-γ-positive cells). Similarly, DCs treated with LPS plus PGE2 allowed 14.6% ± 8.0% of naive T cells to differentiate into TH1 cells. However, when LPS was used in association with ApoC, a high percentage of IFN-γ-producing cells was generated (29.8% ± 17.4%). Moreover, when the TH2 response was measured by detection of IL-4-secreting T cells, we observed a high percentage of IL-4-secreting T cells when they were stimulated with DCs matured in the presence of PGE2 plus TNF-α or LPS (13.6% ± 2.3% and 13.3% ± 0.4%, respectively; Figure 6A). Conversely, lower percentages of IL-4-secreting T cells were observed on stimulation with DCs matured in the presence of apoptotic cells (8.1% ± 0.6% and 8.2% ± 0.4%, respectively, for TNF- and LPS-matured DCs; Figure 6B). Thus, in contrast to PGE2, ApoC allows the migration toward LN chemokines of bona fide TH1-inducing DCs.
ApoC plus LPS allows the migration of DCs that polarize naive T cells toward TH1 response. DCs exposed to apoptotic Jurkat cells (DC/ApoC ratio of 1:8) or PGE2 (3.5 μg/mL) were incubated for 2 days with TNF-α (250 U/mL) or LPS (5 μg/mL). DCs migrating toward CCL21 were mixed in X-VIVO media at a 1:40 ratio with allogeneic naive CD4 T cells. Between day 10 and day 13, T cells were harvested, stained intracellularly for IFN-γ or IL-4, and analyzed by flow cytometry. (A) Percentage of T cells positive for intracellular IFN-γ (mean ± SD of 3 experiments) and FACS histograms of a representative experiment. (B) Percentage of T cells positive for intracellular IL-4 (mean ± SD of 2 experiments) and FACS histograms of a representative experiment. Percentages of IFN-γ- or IL-4-positive cells as defined using isotype control are given.
ApoC plus LPS allows the migration of DCs that polarize naive T cells toward TH1 response. DCs exposed to apoptotic Jurkat cells (DC/ApoC ratio of 1:8) or PGE2 (3.5 μg/mL) were incubated for 2 days with TNF-α (250 U/mL) or LPS (5 μg/mL). DCs migrating toward CCL21 were mixed in X-VIVO media at a 1:40 ratio with allogeneic naive CD4 T cells. Between day 10 and day 13, T cells were harvested, stained intracellularly for IFN-γ or IL-4, and analyzed by flow cytometry. (A) Percentage of T cells positive for intracellular IFN-γ (mean ± SD of 3 experiments) and FACS histograms of a representative experiment. (B) Percentage of T cells positive for intracellular IL-4 (mean ± SD of 2 experiments) and FACS histograms of a representative experiment. Percentages of IFN-γ- or IL-4-positive cells as defined using isotype control are given.
Discussion
DCs are the only cell type able to activate naive T cells, which serves as a rationale for several DC-based antitumoral therapeutic vaccines. Although the effect of the different antigen-loading protocols on the antigen-presentation capacities of DCs have been extensively documented,9-14 the effects of such treatments on this migratory property have received less attention.
In this work, we investigated the role of ApoC interaction on the migratory capacities of DCs toward LN-secreted chemokines. Because the thermosensitive iC3b component of the complement appears to be an important factor in ApoC engulfment38 and can trigger weak CCR7 expression on DCs,24 migration experiments were conducted in autologous complete serum. Under these conditions, exposure to ApoC alone, in the absence of the maturation signal, resulted in only a slight increase of CCR7 expression and promoted the migration of a small fraction of mature DCs (data not shown) in response to LN-directing chemokines. Similarly, TNF-α alone did not provoke important LN chemokine-oriented migration. In contrast, we found that DCs exposed to ApoC and matured with various stimuli showed an enhanced aptitude for migration toward LN chemokines. The uniqueness of this ApoC stimulus is emphasized by several factors: (1) necrotic cells have no effect on DC migration; (2) the mechanism of action of ApoC is different from that of the well-known migration-inducing factor PGE2 because ApoC and PGE2 act synergistically on DC migration; and (3) in contrast to PGE2, the effect of ApoC is down-regulated when heat-inactivated serum—depleted of iC3b, a component involved in ApoC uptake—is used and down-regulated in the absence of CD36, an apoptotic cell receptor.
The action of ApoC on DC migration was not correlated here with ApoC internalization, in agreement with earlier studies on ApoC interaction with macrophages.34,39 Our results also highlight the fact that CCR7 expression is not the sole determinant of DC migration. As a likely explanation of our results, ApoC interaction might promote the acquisition of highly active migratory functions in response to LN chemokines through a modification of CCR7 signaling, as reported with PGE2.30,40
It is generally accepted that ApoC has a null or an inhibitory effect on DC maturation, in contrast to necrotic cells.35 In our experimental settings, the response of DCs to maturation stimuli was not affected by the presence of ApoC, indicating that ApoC-loaded DCs were still susceptible to maturation stimuli, as previously reported.17,41-45 It can thus be presumed that DCs that encounter danger signals can overcome the suppressive effects of ApoC and can trigger an immune response against tumor antigens in the ApoC preparation.25 A positive influence of ApoC interaction on DC migration was recently reported by Ip and Lau.32 These authors showed that interaction with ApoC alone can induce DC migration toward CCL19; 5% of the DCs acquired migratory capacities, in agreement with our own data. However, we observed under these conditions an enrichment of mature DCs in the migrating fraction (data not shown), leading us to conclude that ApoC interaction allows the migration of mature DCs. Our results further demonstrate that ApoC interaction acts in synergy with inflammation stimuli and licenses monocyte-derived DCs for efficient migration toward LN-directing chemokines. Migrating DCs reach 18% of total under these conditions. In addition to the findings of Ip and Lau,32 we show here that DCs require the conjunction of ApoC interaction and maturation signaling to migrate and activate naive T cells.
Given that incompletely matured Langerhans cells have been observed in skin lesion-draining LNs in humans,36 and to ascertain that our migrating DCs are not the “semimature” tolerogenic DCs postulated by Lutz and Schuler,46 we assessed the capacities of our migrating DCs to activate naive T cells toward a TH1 phenotype. PGE2 has been shown to skew DCs to induce a TH2 type of response.31 Previous reports have addressed the role of PGE2 on DC migration and antigen presentation in bulk DC fractions.29,30 Because migrating DCs amount to 20% of total, it appeared essential to analyze phenotypic and functional maturation in these LN-migrating cells, and we assayed here the antigen-presentation properties to naive T cells using the fraction of DCs that migrated toward CCL21. We found that ApoC- and LPS-treated migrating DCs favor the induction of a TH1 response, in contrast to DCs treated with PGE2. Altogether we show here that in addition to triggering migration toward LN chemokines, ApoC interaction can induce a TH1 response in conjunction with DCs matured with strong TH1 stimuli such as LPS. In a recent antitumor vaccine trial reported by Schuler-Thurner et al,11 DCs loaded with a set of HLA class 1- and HLA class 2-restricted peptides and matured with a cytokine cocktail containing TNF-α and PGE2 allowed the development of TH1 responses in vivo, in contrast to in vitro data.30,31 We show here that treatment with apoptotic cells plus selected maturation signals should improve the efficacy of an antitumoral DC-based vaccine by providing a variety of HLA class I and HLA class II antigens, potent DC migration to LNs, and induction of TH1 immune responses.
The actual knowledge of DC functions is continuously being translated into clinical applications. We believe that refinements in antigen-loading procedures may have profound consequences for the success of cancer immunotherapy trials in which DCs are used as natural vaccine adjuvants (for a review, see Schuler et al8 ). In antitumor vaccination protocols, various methods, such as antigenic peptides,11 tumor lysates,14 and whole necrotic or apoptotic tumor cells, have been used for antigen loading of DCs.13 All these methods have been evaluated for their capacity to allow the presentation of tumor antigens through the HLA class I pathway. We optimized the protocols by taking into account two other decisive parameters, the number of DCs that can actually reach the LNs and the capacity of these selected migratory DCs to induce a TH1 response. Our results suggest that, according to these two parameters, loading DCs with apoptotic tumor cells fulfills the most important functional criteria required to elicit efficient cytotoxic responses against tumor antigens.
Prepublished online as Blood First Edition Paper, May 17, 2005; DOI 10.1182/blood-2004-10-3991.
Supported by the Association Cantonale et Intercommunale de Lutte contre le Cancer (ACIC) Baud, la Ligue Contre le Cancer, and Fondation pour la Recherche Medicale (FRM).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Stephane Robert and Ellen Etesse for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal