Abstract
Intrathymic T-cell maturation critically depends on the selective expansion of thymocytes expressing a functionally rearranged T-cell receptor (TCR) β chain. In addition, TCR-independent signals also contribute to normal T-cell development. It is unclear whether and how signals from the 2 types of pathways are integrated. Here, we show that T-cell factor-1 (TCF-1), a nuclear effector of the canonical wingless/int (wnt)/catenin signaling pathway, ensures the survival of proliferating, pre-TCR+ thymocytes. The survival of pre-TCR+ thymocytes requires the presence of the N-terminal catenin-binding domain in TCF-1. This domain can bind the transcriptional coactivator β-catenin and may also bind γ-catenin (plakoglobin). However, in the absence of γ-catenin, T-cell development is normal, supporting a role for β-catenin. Signaling competent β-catenin is present prior to and thus arises independently from pre-TCR signaling and does not substantially increase on pre-TCR signaling. In contrast, pre-TCR signaling significantly induces TCF-1 expression. This coincides with the activation of a wnt/catenin/TCF reporter transgene in vivo. Collectively, these data suggest that efficient TCF-dependent transcription requires that pre-TCR signaling induces TCF-1 expression, whereas wnt signals may provide the coactivator such as β-catenin. The 2 pathways thus have to cooperate to ensure thymocyte survival at the pre-TCR stage. (Blood. 2005;106:1726-1733)
Introduction
Key events of T-cell maturation are dependent on the T-cell receptor (TCR) for antigen. An early stage of intrathymic T-cell development requires a functionally rearranged TCR β chain, which is part of an immature TCR, the so-called pre-TCR complex. This complex not only is required to trigger thymocyte cycling but also ensures their survival and induces the differentiation from the DN (double negative, CD4-CD8-) to the DP (double positive, CD4+CD8+) stage of thymocyte development. Development past the DP stage depends on a mature αβTCR with proper affinity for peptide/major histocompatibility complex (MHC) class I or II.
Besides the TCR, additional TCR-independent pathways are required for normal T-cell development. For instance, prior to TCR-dependent stages Notch-1 signaling mediates the commitment of early intrathymic progenitors to the T-cell lineage, whereas interleukin 7 (IL-7) and SCF (stem cell factor) mediate precursor expansion and survival. At subsequent stages of development the formation of a pre-TCR is crucial for further development. Yet it is not clear whether additional TCR-independent signaling pathways contribute and how signals of such pathways would be coordinated with signals from the pre-TCR. To address this issue we have studied the precise role of the nuclear effector of the canonical wingless/int (wnt) signaling pathway T-cell factor-1 (TCF-1): TCF-1 deficiency results in a partial block of thymocyte development at the pre-TCR stage,1-3 raising the possibility that pre-TCR and wnt signals are coordinated.
The cellular and molecular consequences of wnt signaling have been defined in some detail in epithelial stem cells and their progenitors, especially those of the colon.4 The general scheme of the canonical wnt pathway is that the exposure of cells to wnt protein inhibits the intracellular degradation of β-catenin. The accumulation of signaling-competent β-catenin allows the association with DNA-binding factors of the TCF/LEF (lymphoid enhancer factor) family and induces the transcription of downstream target genes. In contrast to epithelial cells, relatively little and somewhat controversial information is available regarding the role and importance of this pathway for T-cell development.
Several wnt proteins and their Frizzled (Fz)/low-density lipoprotein receptor-related protein 5/6 (LRP5/6) receptor(s) are expressed in the thymic epithelial cells and thymocytes, respectively.5 Thymocyte numbers are reduced in mice lacking wnt-1 and wnt-4,6 and the expression of soluble Fz receptors reduces DN to DP transition in fetal thymocytes.7 In adult mice, the inducible overexpression of a dominant-interfering form of axin, which is part of the β-catenin degradation complex, results in massive apoptosis of cortical thymocytes.8 Finally, the conditional deletion of β-catenin partially inhibits T-cell development past the pre-TCR stage,9 although a second study, using independently generated conditional β-catenin knock-out mice, has questioned the role for β-catenin in thymocyte development.10 This raised the possibility that the β-catenin homologue γ-catenin (plakoglobin) might play a role for T-cell development.
With regard to the nuclear effectors of wnt-signaling, TCF-1 is the only limiting TCF/LEF family factor during T-cell development. TCF-1 deficiency results in multiple distinct, incomplete thymocyte developmental blocks.1,2 During the DP stage, LEF-1 can partially replace TCF-1 function, since DP thymocytes are completely absent in LEF-1 null mice with a hypomorphic TCF-1 allele.11 In the absence of TCF-1, quiescent DP thymocytes undergo very rapid spontaneous death.3 However, the role of TCF-1 at the pre-TCR checkpoint, where cells expressing a properly rearranged TCRβ chain normally begin to cycle, accumulate, and differentiate, has not been determined.
We found that thymocytes lacking TCF-1 formed a functional pre-TCR as cycling was properly initiated, but such thymocytes underwent rapid cell death. Thymocyte survival at the pre-TCR stage required the presence of the catenin-binding domain in TCF-1, yet was independent of γ-catenin, suggesting that the TCF-1-dependent effect is mediated at least in part by β-catenin. Whereas signaling competent β-catenin arises independent of pre-TCR signaling, TCF-1 is highly induced on pre-TCR signaling. These findings suggest that the pre-TCR and canonical wnt signals have to cooperate to ensure thymocyte survival.
Materials and methods
Mice
C57BL/6 (B6), CD45.1 congenic B6, and recombination activating gene-1 (RAG-1)-deficient mice were purchased from Harlan Olac (Horst, The Netherlands) and the Jackson Laboratories (Bar Harbor, ME), respectively. TCF-1-deficient (exon VII) and β-catenin-activated transgene driving expression of nuclear β-galactosidase (BAT-gal) reporter mice have been described.1,12 TCF-1-/-RAG-1-/- mice were obtained by breeding.
RAG-1-deficient mice were injected intraperitoneally with 100 μg anti-CD3ϵ monoclonal antibody (mAb; 145-2C11, LICR).
Typing
TCF-1-deficient mice were identified by polymerase chain reaction (PCR).13 RAG-1-deficient mice were identified by PCR using the following primers: RAG sense, CCA GTA GAT ACC ATT GCG AAG AGG; RAG anti, CAC GTT CTG TGAACC ATG CTC TAT C; and Neo anti, CCG CTT CCA TTG CTC AGC GG.
Fetal liver chimeras
γ-Catenin+/- mice,14 backcrossed more than 10 times to B6 (CD45.2+), were intercrossed. Embryos were obtained at day 12.5 of gestation and were genotyped by PCR as described.14 Recipient B6 CD45.1+ mice were lethally irradiated (9.6 Gy [960 rad], 137-Cs source) before intravenous injection of 5 × 106 fetal liver cells. Chimeras were analyzed 3 to 5 months after reconstitution.
Flow cytometry
MAbs to CD4 (GK1.5), CD8 (53.6.7), CD117 (ACK2), CD127 (A7R34), macrophage antigen-1 (Mac-1; M1/70), B220 (RA3-6B2), granulocyte-differentiation antigen (Gr1; RB6-8C5), and Ter119 were conjugated at the LICR. MAbs to TCRβ (H57), CD3 (17A2), TCRγδ (GL3), and natural killer 1.1 (NK1.1; PK136) were from BD Pharmingen (San Diego, CA).
Thymocytes were obtained from 4- to 8-week-old mice. Crystalline fragment (Fc) receptors were blocked by preincubation with 2.4G2 (anti-CD16/32) hybridoma supernatant. Thymocytes were labeled with mAbs for 4-color flow cytometry. A cocktail of fluorescein isothiocyanate (FITC)-conjugated anti-TCRβ, TCRγδ, CD3ϵ, CD4, CD8, Mac-1, B220, NK1.1, Ter119, Gr1 mAbs was used to identify lineage-negative (lin-)DN cells. Intracellular staining with mAbs to TCRβ, TCRγδ, or CD3ϵ was performed as described.15 Samples were run on a FACSCalibur flow cytometer and analyzed with Cell Quest software (Becton Dickinson, San Jose, CA).
For wnt reporter assays, DN cells were enriched via complement-dependent lysis using RL172.4 (anti-CD4) and 3.168.8.1 (anti-CD8) mAbs, stained with C12FDG (33 μM; Molecular Probes, Leiden, The Netherlands) 40 minutes at room temperature in RPMI + 10% fetal calf serum (FCS), transferred on ice, and stained with anti-CD25, anti-CD44 Abs. Contaminating cells were gated out using a cocktail of biotinylated mAbs.
To determine the DNA content, surface-labeled and paraformaldehyde-fixed cells were stained for 5 minutes at room temperature with Hoechst 33342 (Molecular Probes) in phosphate-buffered saline (PBS)/3% FCS/0.5% saponin. Cells were run on an LSR flow cytometer (Becton Dickinson, San Jose, CA) using the doublet discrimination unit.
To identify preapoptotic cells, surface-stained thymocytes were resuspended in 1 × binding buffer (106 cells/mL) and stained for 10 minutes with Annexin V-cyanine 5 (Cy5).
Bromodeoxyuridine (BrdU) incorporation
Mice were injected intraperitoneally with 1.8 mg BrdU in PBS. After surface staining, thymocytes were fixed and permeabilized. After washing, cells were treated with 50 μL DNAse for 1 hour at 37°C. After washing the cells were stained for 20 minutes at room temperature with anti-BrdU-FITC mAb (BrdU Flow kit; BD Pharmingen).
Survival kinetics
Thymocytes were incubated at 37°C in 96-well plates at a concentration of 106 cells per 200 μL incomplete Dulbecco Modified Eagle Medium (DMEM) containing 10% FCS, 2 mM glutamine, 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 100 U/mL streptomycin. The cells were collected after various time points and surface stained as described in “Flow cytometry.” A fixed number of microspheres (bacteria counting kit; Molecular Probes) were added to each sample. Thymocytes and microspheres were discriminated based on forward scatter (FSC) and side scatter (SSC). Dead cells were further excluded based on 7-amino-actinomycin D (7-AAD) uptake (Molecular Probes). The survival of DN cells was determined by comparing the ratio of beads to viable cells at each time point of the culture. The ratio obtained at the onset of the culture was assigned 100%.
Western blot
Western blots on total cell lysates (106 cells) were performed as described.3 Blots were incubated overnight at 4°C with primary antibodies: rabbit anti-TCF-1 (kindly provided by Y. Katsura, Kyoto, Japan), anti-active β-catenin (8E7; Upstate, Charlottesville, VA), anti-β-catenin (clone 14), and anti-γ-catenin (clone 15; BD Transduction Laboratories, Lexington, KY) or anti-α-tubulin (B-5-1-2; Sigma, St Louis, MO). Western blots were revealed with horseradish peroxidase (HRP)-conjugated anti-mouse and -rabbit Abs (Sigma) followed by enhanced chemiluminescence detection (Pierce, Rockford, IL).
Quantitative RT-PCR
SYBR green quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using a Light Cycler (Roche, Rotkreuz, Switzerland). Total RNA was extracted using RNeasy QIAGEN kit (QIAGEN, Hilden, Germany), and cDNA was synthesized using oligo(dT) (Amersham Biosciences, Otelfingen, Switzerland). RNA was digested using RNAse ONE (Promega, Madison, WI), and cDNA was purified using QIAGEN DNA purification kit. The primer sequences were as follows: TATA-binding protein (TBP) sense, ACT TCG TGC AAG AAA TGC TGA A; TBP antisense, TGT CCG TGG CTC TCT TAT TCT CA; Tcf-1 sense, CGC TGC ATA ACA AGC C; Tcf-1 antisense, CCA GCT CAC AGT ATG GG; β-catenin sense, GCA GCG ACT AAG CAG G; β-catenin antisense, GGA CGG TGC GTA CAA GA. Amplification plots were analyzed using the second derivative method with LC data analysis 3.5 software (Roche). Corrections for amplification efficiency were included.
Statistics
Differences between data sets were determined using the 2-tailed t test. Differences were considered statistically significant when the P value was less than .05.
Results
Thymocyte maturation in TCF-1-deficient mice
TCF-1-deficient thymi contain 10- to 20-fold fewer thymocytes as compared with wild type (Figure 1A-B). This is due to the lack of TCF-1 in thymocytes and not their environment (data not shown).2 Decreased thymic cellularity is predominantly due to a reduction in DP cells (Figure 1A-B), which undergo rapid spontaneous cell death.3 However, because the restoration of DP survival did not restore normal thymocyte numbers,3 we have analyzed in more detail the phenotype of DN thymocytes, where thymocyte expansion is initiated. We assessed CD44 and CD25 expression in a population of cells, which were negatively gated for a series of markers defining mature cells (lineage markers, ie, CD3, B220, CD4, CD8, CD11b, TCRαβ, TCRγδ, NK1.1, Gr1, Ter119 termed “lin-”).
Thymocyte development in TCF-1-deficient mice. (A) Density plots show a representative analysis of CD4/CD8 and CD44/CD25 subsets among lin- thymocytes (cells not expressing CD4, CD8, CD3, B220, CD11b, TCRβ, TCRγδ, NK1.1, Gr1, and Ter119). Numbers indicate the percentage of cells in the respective gate. (B) Bar graphs show the mean ± SD of the absolute number of cells of the indicated type; DN1 indicates lin- CD44+CD25-/low, DN2, lin- CD44+CD25hi; DN3, lin- CD44-CD25hi; DN4, lin- CD44-CD25neg. Histograms show the expression of CD117 (c-kit) and CD127 (IL-7 receptor α [IL-7Rα]) (C), intracellular TCRβ (D), or CD3ϵ (E) and TCRγδ (F) in the indicated lin- population. Numbers indicate the percentage of cells in the regions defined by horizontal bars. (G) Bar graphs show the mean of the absolute number of cells of the indicated type (± SD).
Thymocyte development in TCF-1-deficient mice. (A) Density plots show a representative analysis of CD4/CD8 and CD44/CD25 subsets among lin- thymocytes (cells not expressing CD4, CD8, CD3, B220, CD11b, TCRβ, TCRγδ, NK1.1, Gr1, and Ter119). Numbers indicate the percentage of cells in the respective gate. (B) Bar graphs show the mean ± SD of the absolute number of cells of the indicated type; DN1 indicates lin- CD44+CD25-/low, DN2, lin- CD44+CD25hi; DN3, lin- CD44-CD25hi; DN4, lin- CD44-CD25neg. Histograms show the expression of CD117 (c-kit) and CD127 (IL-7 receptor α [IL-7Rα]) (C), intracellular TCRβ (D), or CD3ϵ (E) and TCRγδ (F) in the indicated lin- population. Numbers indicate the percentage of cells in the regions defined by horizontal bars. (G) Bar graphs show the mean of the absolute number of cells of the indicated type (± SD).
TCF-1-deficient and -sufficient mice have comparable numbers of lin- DN1 cells (CD44+CD25-) (Figure 1A-B). However, in contrast to wild type (63%), TCF-1-deficient lin- DN1 contain few cells (9%) expressing high levels of CD117 (Figure 1C), which defines early T-lineage progenitors (ETPs).16 Thus, the phenotype of TCF-1-deficient mice may be related in part to a reduced influx of progenitor cells from the bone marrow. TCF-1-deficient DN1 cells are predominantly CD127+ and express intermediate levels of CD117 (Figure 1C). The former is consistent with the phenotype of late DN1 as well as DN2 cells.17 Indeed, many TCF-1-deficient DN1 cells express low levels of CD25. On the other hand bona fide CD25hi DN2 cells are almost completely absent (Figure 1A-B). DN3 cells (CD25+CD44-) were present even though at significantly reduced numbers as compared to wild-type animals (Figure 1A-B). Some DN4 cells (CD25-CD44-) were detectable in 3-week-old mice but almost completely absent thereafter (Figure 1A-B). Thus, in addition to a depletion of DP cells, TCF-1-deficient mice display developmental blocks at the DN1 (CD25-CD44+) and DN3 stages. These data confirm and extend previous analyses, which were based on less stringent exclusion of lin+ cells.2,3
Generation and function of the pre-TCR in TCF-1-deficient mice
The absence of TCF-1 impairs the DN3 to DN4 transition, where TCRβ expression normally results in a large number of proliferating cells. To test whether TCF-1-/- mice contained a functional pre-TCR, we investigated intracellular (ic) TCRβ expression. In normal mice, icTCRβ expression is detected in 20% to 30% of DN3 cells using flow cytometry (Figure 1D). A comparable fraction of DN3 cells expressed icTCRβ in TCF-1-/- mice, yet absolute numbers were reduced as compared with wild type (Figure 1G). At the subsequent stage of development, icTCRβ+ cells accumulate and constitute 80% to 90% of wild-type DN4 cells, compared with only 29.6% in TCF-1-deficient mice. Virtually all DN3 and DN4 cells expressed icCD3ϵ, demonstrating that these are T lineage cells (Figure 1E), yet they were not TCRγδ lineage cells (Figure 1F). Thus, in the absence of TCF-1, CD3ϵ and TCRβ are properly expressed, but icTCRβ+ cells fail to accumulate (Figure 1E).
Cycling, differentiation, and turn over of TCF-1-deficient thymocytes
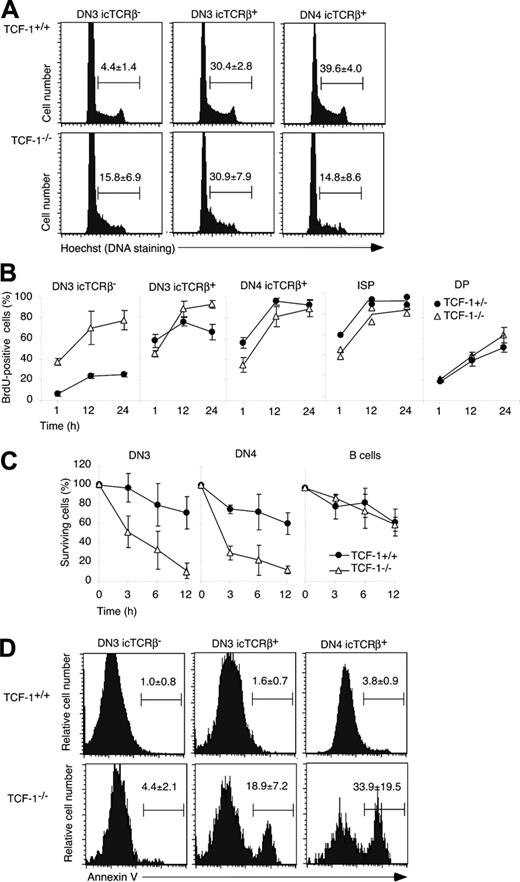
The formation of the pre-TCR complex triggers thymocyte cycling. We thus measured the DNA content of the different thymocyte subsets using Hoechst staining (Figure 2A). In TCF-1+/- (data not shown) or TCF-1+/+ mice, few DN3 icTCRβ- cells (4.4% ± 1.4%) were in the S/G2/M phase of the cell cycle, whereas 30.4% ± 2.8% of icTCRβ+ DN3 cells were in cycle. Among TCF-1-deficient icTCRβ+ DN3 thymocytes a comparable fraction of thymocytes (30.9% ± 7.9%) was in cycle. However, while cycling was sustained in DN4 icTCRβ+ cells of normal mice (39.6% ± 4.0%), only 14.8% ± 8.6% of the corresponding TCF-1-deficient cells were in cycle (Figure 2A). Finally, compared with DN4 icTCRβ+, cycling among DN4 icTCRβ- cells was considerably lower in TCF-1-sufficient (10.1 ± 1.3) and -deficient mice (4.3 ± 2.9). Of note, TCF-1-deficient thymocytes yield a normal Hoechst profile, and no apparent underrepresentation of cells in G2/M phase when Hoechst staining is performed in the absence of icTCRβ staining (data not shown). These results show that DN3 TCF-1-deficient thymocytes, which express TCRβ cycle as efficiently as the corresponding wild-type cells, yet cycling is not sustained at the DN4 stage.
Cycling, turnover, and survival of TCF-1-deficient DN cells. (A) Thymocytes were stained with a lin cocktail, including CD44, for CD25, and icTCRβ. Histograms show the relative DNA content among the indicated DN subsets, as determined by Hoechst staining. Numbers represent the mean of cells in S/G2/M phase of the cell cycle (± SD). Of note, TCF-1-deficient thymocytes yield a normal Hoechst profile and no apparent underrepresentation of cells in G2/M phase when Hoechst staining is performed in the absence of icTCRβ staining (data not shown). (B) Mice were injected intraperitoneally with the nucleotide analog BrdU and put on drinking water containing BrdU. Thymocytes were collected at the indicated time points and stained with antibodies to CD4, CD8, TCRβ, and BrdU or the lineage cocktail (including CD44), CD25, icTCRβ, and BrdU. The graph depicts the mean percentage (± SD) of BrdU+ cells in the indicated thymocyte subset. (C) Total thymocytes were incubated for the indicated periods of time in complete culture medium (without the addition of growth factors). After collection they were stained with antibodies to the lineage cocktail, CD44 and CD25. For each time point, the presence of viable DN3 or DN4 was estimated relative to a fixed number of added microspheres. The ratio of DN3 or (DN4) to beads at the onset of the culture was set to 100%. The graphs show the mean percentage (± SD) of surviving cells relative to the onset of the culture. (D) Thymocytes were surface stained for CD25, the lin cocktail (including CD44), and annexin V. After fixation and permeabilization, thymocytes were stained for icTCRβ. Histograms show the mean percentage of annexin V-positive cells (± SD) in each of the indicated DN subset.
Cycling, turnover, and survival of TCF-1-deficient DN cells. (A) Thymocytes were stained with a lin cocktail, including CD44, for CD25, and icTCRβ. Histograms show the relative DNA content among the indicated DN subsets, as determined by Hoechst staining. Numbers represent the mean of cells in S/G2/M phase of the cell cycle (± SD). Of note, TCF-1-deficient thymocytes yield a normal Hoechst profile and no apparent underrepresentation of cells in G2/M phase when Hoechst staining is performed in the absence of icTCRβ staining (data not shown). (B) Mice were injected intraperitoneally with the nucleotide analog BrdU and put on drinking water containing BrdU. Thymocytes were collected at the indicated time points and stained with antibodies to CD4, CD8, TCRβ, and BrdU or the lineage cocktail (including CD44), CD25, icTCRβ, and BrdU. The graph depicts the mean percentage (± SD) of BrdU+ cells in the indicated thymocyte subset. (C) Total thymocytes were incubated for the indicated periods of time in complete culture medium (without the addition of growth factors). After collection they were stained with antibodies to the lineage cocktail, CD44 and CD25. For each time point, the presence of viable DN3 or DN4 was estimated relative to a fixed number of added microspheres. The ratio of DN3 or (DN4) to beads at the onset of the culture was set to 100%. The graphs show the mean percentage (± SD) of surviving cells relative to the onset of the culture. (D) Thymocytes were surface stained for CD25, the lin cocktail (including CD44), and annexin V. After fixation and permeabilization, thymocytes were stained for icTCRβ. Histograms show the mean percentage of annexin V-positive cells (± SD) in each of the indicated DN subset.
We also noted that in the absence of TCF-1, a significant fraction (15.8% ± 6.9%) of DN3 cells lacking icTCRβ were in cycle (Figure 2A). Thus, some TCF-1-deficient thymocytes fail to properly arrest at G1 before TCRβ rearrangement, raising the possibility that TCF-1 represses cell-cycle progression prior to pre-TCR signaling.
In addition to cycling, pre-TCR signaling drives differentiation of thymocytes. To follow these events, mice were exposed to BrdU to establish the kinetics of incorporation of BrdU into thymocyte DNA in vivo. The presence of BrdU+ cells after a 1-hour pulse shows that TCF-1-/- thymocytes efficiently cycle (Figure 2B). The DN3 icTCRβ- cells incorporated BrdU significantly faster than wild-type mice, confirming our observation with Hoechst staining. Upon the expression of icTCRβ the abundance of BrdU-positive cells increased similarly in TCF-1-deficient and -sufficient thymocytes. Finally, BrdU+ DP cells accumulated at a comparable rate (Figure 2B). Since quiescent DP cells derive from proliferating immature single positive (ISP) cells, these findings suggest that TCF-1 deficiency does not alter the kinetics of the ISP to DP differentiation. In addition, these findings independently confirm that TCF-1-deficient thymocytes have the capacity to rapidly cycle.
Impaired survival of TCF-1-deficient thymocytes
Despite efficient cycling of icTCRβ+ DN3 cells, DN4 cells do not accumulate in TCF-1-deficient mice. We thus tested whether these cells are prone to apoptosis despite being icTCRβ+. To this end, DN3 and DN4 thymocytes from TCF-1-deficient mice were cultured at 37°C in the absence of exogenous growth factors. Within 3 hours of culture, 50% and 70% of viable DN3 or DN4, respectively, were lost from the culture of TCF-1-deficient thymocytes (Figure 2C). In comparison only 5% and 25% of the corresponding TCF-1+/+ (Figure 2C) or TCF-1+/- (data not shown) thymocytes were lost.
To independently confirm these analyses, we stained freshly isolated DN3 and DN4 cells with Annexin V, which recognizes phosphatidylserine expressed on the surface of preapoptotic cells. This analysis was combined with icTCRβ staining. Few DN3 or DN4 cells (< 4%) were Annexin V positive in TCF-1+/+ (Figure 2D) or TCF-1+/- mice (data not shown). However, a substantial fraction of TCF-1-deficient icTCRβ+ DN3 and DN4 cells were Annexin V positive (18.9% ± 7.2% and 33.9% ± 19.5%, respectively). In contrast, significantly fewer icTCRβ-negative DN3 cells were Annexin positive (4.4% ± 2.1%) in TCF-1-deficient mice (Figure 2D). Thus, the fraction of Annexin V-positive cells considerably increased upon the expression of icTCRβ. These findings suggest that pre-TCR signaling induces cycling, but that in the absence of TCF-1 the survival of TCRβ+ cells is not ensured.
Testing pre-TCR signaling in TCF-1-deficient mice
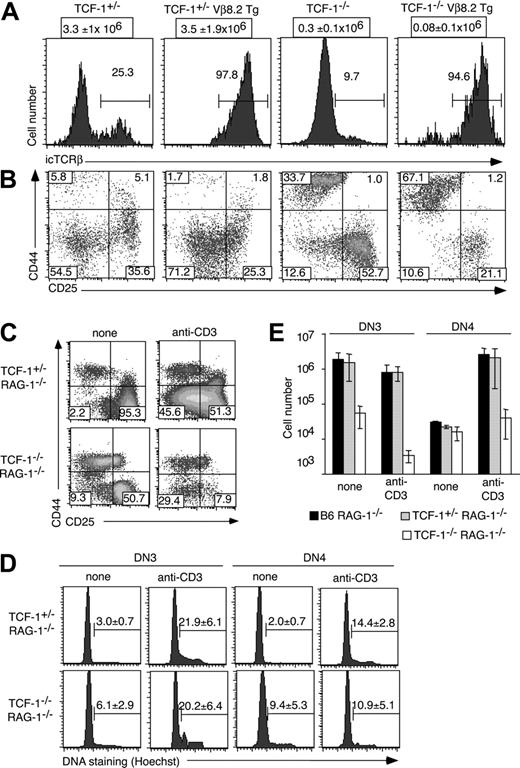
We functionally tested whether the apparent arrest at the pre-TCR stage was due to the death of cells expressing a functionally rearranged TCRβ locus or alternatively related to inefficient TCRβ rearrangement/expression. Thus, we generated TCF-1-/- mice transgenic (Tg) for a rearranged TCRVβ8.2 chain (Figure 3A). The number of DN3 cells in TCF-1-/- Vβ8.2 Tg mice was further reduced as compared with TCF-1-/- mice (Figure 3B); thus, the expression of a TCRβ Tg aggravates rather than ameliorates T-cell development in TCF-1-deficient mice. This is supporting the view that in the absence of TCF-1, pre-TCR signaling is driving thymocytes into apoptosis. In contrast to the apoptosis, which is induced upon TCRβ expression, we obtained no evidence that the mature TCRαβ is inducing the death of TCF-1-deficient DP thymocytes. This is based on the finding that the DP cellularity in TCRα/TCF-1 double-deficient mice was as low as that of TCF-1-deficient mice (data not shown).
Induction of pre-TCR signaling in TCF-1/RAG-1 double-deficient mice
To independently test whether TCF-1 ensured the survival of thymocytes receiving a pre-TCR signal, we generated TCF-1/RAG-1 double-deficient mice. Like RAG-1-deficient thymocytes, thymocyte development in TCF-1/RAG-1 double-deficient mice was arrested at the DN3 stage (Figure 3C). However, TCF-1-/-RAG-1-/- mice contained significantly fewer DN as compared with TCF-1+/-RAG-1-/- mice (Figure 3E).
Thymocyte development in TCRβ Tg and RAG-1-/- TCF-1-deficient mice. (A) Histograms show the expression of icTCRβ in the DN3 subset for each of the indicated mouse strains. Numbers indicate the percentage of cells in the region defined by a horizontal bar. Absolute numbers of DN cells (± SD) are shown above each graph. (B) Density plots show thymocytes from the indicated types of mice, which were surface stained with antibodies to a lineage cocktail, CD44, and CD25. Numbers indicate the percentage of cells in the respective quadrant. (C) The indicated types of mice were injected with anti-CD3 mAb. Twenty-four hours later thymocytes were isolated and stained with the lineage cocktail plus CD44 and CD25. Numbers depict the percentage of cells in the respective quadrant. (D) Thymocytes were stained with a lin cocktail, CD44, and CD25. Histograms show the relative DNA content among the indicated DN subsets, as determined by Hoechst staining. Numbers represent the mean percentage (± SD) of cells in S/G2/M phase of the cell cycle. (E) Bar graphs show the mean of the absolute number (± SD) of DN3 and DN4 cells among lin-negative cells in mice injected with anti-CD3 or in control mice. Note that the number of cells is shown using a log scale.
Thymocyte development in TCRβ Tg and RAG-1-/- TCF-1-deficient mice. (A) Histograms show the expression of icTCRβ in the DN3 subset for each of the indicated mouse strains. Numbers indicate the percentage of cells in the region defined by a horizontal bar. Absolute numbers of DN cells (± SD) are shown above each graph. (B) Density plots show thymocytes from the indicated types of mice, which were surface stained with antibodies to a lineage cocktail, CD44, and CD25. Numbers indicate the percentage of cells in the respective quadrant. (C) The indicated types of mice were injected with anti-CD3 mAb. Twenty-four hours later thymocytes were isolated and stained with the lineage cocktail plus CD44 and CD25. Numbers depict the percentage of cells in the respective quadrant. (D) Thymocytes were stained with a lin cocktail, CD44, and CD25. Histograms show the relative DNA content among the indicated DN subsets, as determined by Hoechst staining. Numbers represent the mean percentage (± SD) of cells in S/G2/M phase of the cell cycle. (E) Bar graphs show the mean of the absolute number (± SD) of DN3 and DN4 cells among lin-negative cells in mice injected with anti-CD3 or in control mice. Note that the number of cells is shown using a log scale.
Pre-TCR signaling was induced by the injection of anti-CD3ϵ mAb, and thymocytes were analyzed 24 hours later. Equivalent proportions of DN3 cells were induced into cycle in TCF-1-/-RAG-1-/- and TCF-1+/-RAG-1-/- mice (Figure 3D). However, whereas thymocyte numbers increased in TCF-1+/-RAG-1-/- mice after anti-CD3ϵ injection, TCF-1-/-RAG-1-/- mice contained significantly fewer DN as compared with noninjected controls (Figure 3E, note that a log scale is used). We conclude that preTCR signaling in TCF-1-/-RAG-1-/- mice induces thymocyte cycling, but that stimulated cells do not survive.
The role of the catenin-binding domain in TCF-1
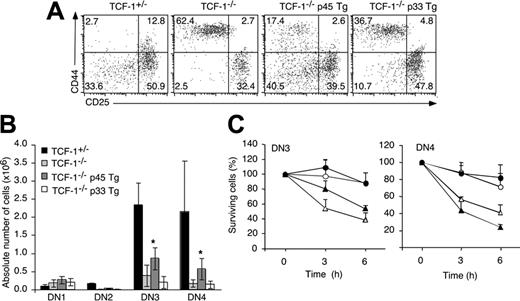
To determine more precisely the role of TCF-1 we tested whether the NH2-terminal catenin-binding domain in TCF-1 was necessary to ensure the survival of preTCR+ thymocytes. This domain is present in p45 TCF-1 Tg but not in p33 TCF-1 Tg mice. Consistent with our previous data, the p45 (but not the p33) TCF-1 Tg partially restores thymocyte numbers in TCF-1-deficient mice.3 At the pre-TCR stage, the presence of lin- DN3 and DN4 thymocytes was significantly improved in TCF-1-/- mice expressing the p45 but not the p33 Tg (Figure 4A-B). As shown in Figure 4C, the catenin-binding domain of TCF-1 is required to ensure thymocyte survival at the DN3 and DN4 stage. Corresponding conclusions were reached when DN3 cells were stained with Annexin V (data not shown). The data imply that the NH2-terminal catenin-binding domain of TCF-1 plays a critical role for the survival of proliferating (DN3 and DN4) as well as quiescent (DP) thymocytes.3
The role of γ-catenin for thymocyte development
The analysis of TCF-1 Tg mice implies a critical role for coactivators associating with the NH2-terminal domain of TCF-1 for T-cell development at the pre-TCR stage. Since the importance of β-catenin for T-cell development is controversial,9,10 we have analyzed whether γ-catenin (plakoglobin) played a role for thymocyte development. Indeed, γ-catenin mRNA (not shown) and protein are readily detectable in thymocytes from RAG-1-deficient mice (Figure 5C).
The null mutation of γ-catenin is lethal between days 12 and 16 of embryogenesis.14 We thus isolated day-12 fetal liver of γ-catenin null, heterozygous, or wild-type embryos to reconstitute lethally irradiated C57BL/6 recipients. Hematopoietic cells of donor and recipient origin were discriminated using the allelic markers CD45.2 (donor) and CD45.1 (recipient).
Pre-TCR signaling in p45 Tg TCF-1-deficient mice. (A) Thymocytes from the indicated types of mice were stained with anti-CD44 and CD25 and the lin cocktail. Numbers depict the percentage of cells in the respective quadrants. (B) Bar graphs show the mean of the absolute number (± SD) of DN1, DN2, DN3, and DN4 cells among lin-negative cells. *Statistically significant difference (P < .05) as compared with TCF-1 knockout. (C) Thymocytes derived from the indicated types of mice were cultured at 37°C in the absence of growth factors. After collection they were stained with antibodies to the lineage cocktail, CD44, and CD25. For each time point the percentage of viable DN3 and DN4 cells was determined relative to a fixed number of added microspheres. The ratio at the onset of culture was arbitrarily set to 100%. The graphs show mean (± SD) percentage of surviving DN3 and DN4 cells relative to the onset of culture. ○ indicates TCF-1+/-; ▵, TCF-1-/-; •, TCF-1-/- p45 Tg; and ▴, TCF-1-/- p33 Tg.
Pre-TCR signaling in p45 Tg TCF-1-deficient mice. (A) Thymocytes from the indicated types of mice were stained with anti-CD44 and CD25 and the lin cocktail. Numbers depict the percentage of cells in the respective quadrants. (B) Bar graphs show the mean of the absolute number (± SD) of DN1, DN2, DN3, and DN4 cells among lin-negative cells. *Statistically significant difference (P < .05) as compared with TCF-1 knockout. (C) Thymocytes derived from the indicated types of mice were cultured at 37°C in the absence of growth factors. After collection they were stained with antibodies to the lineage cocktail, CD44, and CD25. For each time point the percentage of viable DN3 and DN4 cells was determined relative to a fixed number of added microspheres. The ratio at the onset of culture was arbitrarily set to 100%. The graphs show mean (± SD) percentage of surviving DN3 and DN4 cells relative to the onset of culture. ○ indicates TCF-1+/-; ▵, TCF-1-/-; •, TCF-1-/- p45 Tg; and ▴, TCF-1-/- p33 Tg.
γ-Catenin-deficient and -sufficient fetal liver stem cells contributed equally well to the thymus of recipient mice (> 96%) (Figure 6A). Thymocyte number (Figure 6B) and subset distribution according to CD4/CD8 (Figure 6C) or CD44/CD25 among lin- cells (Figure 6D) was not significantly different between wild-type and mutant thymocytes. Moreover, preliminary analyses indicated that thymocyte cycling at the DN3/DN4 stage was comparable (data not shown). In contrast, the survival of γ-catenin null DN3 and DN4 thymocytes was slightly but consistently reduced when compared with the corresponding γ-catenin-sufficient populations (Figure 6E). Yet, unlike TCF-1, γ-catenin-null DP thymocytes survived as well as γ-catenin-sufficient DP cells (Figure 6E). Thus, the absence of γ-catenin does not significantly impair T-cell development, even though a role for γ-catenin at the pre-TCR is apparent in vitro.
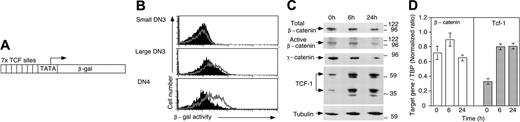
Regulation of TCF-1 and β-catenin levels and TCF/catenin signaling. (A) The wnt reporter transgene consists of 7 consensus TCF-binding sites upstream of a minimal promoter-TATA box, which drive the expression of β-galactosidase (β-gal). (B) Histograms show profiles reflecting β-gal activity in enriched populations of DN3 and DN4 thymocytes from BAT-GAL (gray line) and control mice (filled histograms). Small versus large DN3 cells were gated according to their forward and side scatter characteristics. (C) RAG-1-deficient mice were injected with anti-CD3ϵ mAb, and thymocytes were isolated before (0 hours) or 6 hours and 24 hours later. Total thymocyte lysates, corresponding to 106 cells per lane, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred and subjected to Western blot analysis with the indicated antibodies. Molecular weight markers are indicated at right in kDa. (D) Quantitative RT-PCR analysis for β-catenin and Tcf-1 mRNA. RAG-1-deficient mice were injected with anti-CD3ϵ mAb, mRNA was isolated at the indicated time points and subjected to quantitative PCR (qPCR) analysis. Tcf-1 and β-catenin mRNA levels are indicated relative to that of TBP (TATA-binding protein) as described in “Materials and methods.” Bars show the mean ratio ± SD of triplicate determinations.
Regulation of TCF-1 and β-catenin levels and TCF/catenin signaling. (A) The wnt reporter transgene consists of 7 consensus TCF-binding sites upstream of a minimal promoter-TATA box, which drive the expression of β-galactosidase (β-gal). (B) Histograms show profiles reflecting β-gal activity in enriched populations of DN3 and DN4 thymocytes from BAT-GAL (gray line) and control mice (filled histograms). Small versus large DN3 cells were gated according to their forward and side scatter characteristics. (C) RAG-1-deficient mice were injected with anti-CD3ϵ mAb, and thymocytes were isolated before (0 hours) or 6 hours and 24 hours later. Total thymocyte lysates, corresponding to 106 cells per lane, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred and subjected to Western blot analysis with the indicated antibodies. Molecular weight markers are indicated at right in kDa. (D) Quantitative RT-PCR analysis for β-catenin and Tcf-1 mRNA. RAG-1-deficient mice were injected with anti-CD3ϵ mAb, mRNA was isolated at the indicated time points and subjected to quantitative PCR (qPCR) analysis. Tcf-1 and β-catenin mRNA levels are indicated relative to that of TBP (TATA-binding protein) as described in “Materials and methods.” Bars show the mean ratio ± SD of triplicate determinations.
Pre-TCR and wnt signaling
On the basis of the genetic data we next addressed whether there was a relation between pre-TCR and TCF/catenin signaling. To this end we used BAT-gal reporter mice in which the expression of β-galactosidase is under the control of multimerized TCF/LEF-binding sites (Figure 5A) and β-galactosidase expression reports wnt activity in vivo.12 Thymocytes from BAT-gal or nontransgenic control mice were loaded with a galactosidase substrate. Galactosidase activity was apparent in blasting DN3 cells displaying an enlarged FSC/SSC profile, that is, in cells that have received a pre-TCR signal but was not detected in small quiescent DN3 cells. The signal was further increased in DN4 cells (Figure 5B). These findings suggest that TCF/catenin activity coincides with pre-TCR signaling.
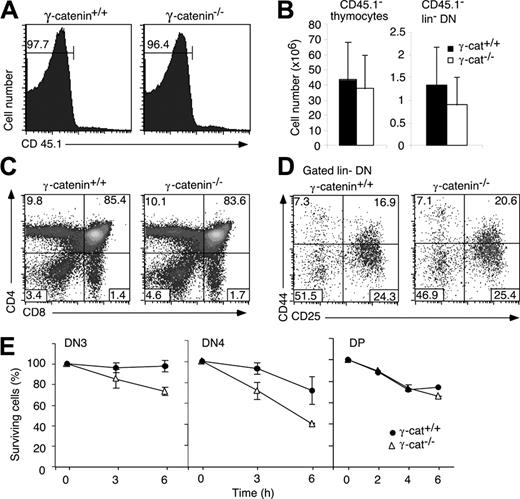
T-cell development in the absence of γ-catenin. (A) Histograms show thymocytes derived from lethally irradiated adult CD45.1+ recipient mice, which were reconstituted with CD45.2+ fetal liver cells of the indicated γ-catenin genotype. Numbers indicate the percentage of CD45.1- (donor-derived) cells. (B) Bar graphs represent the mean (± SD) of the absolute number of CD45.1- total and lin- thymocytes of 5 chimeras. Dot plots show CD45.1- thymocytes stained with CD4 and CD8 (C) or with the lineage cocktail, CD44 and CD25 (D). Numbers indicate the percentage of cells in the respective quadrants. (E) Total thymocytes were incubated for the indicated periods of time in complete culture medium (without the addition of growth factors). After collection, the cells were stained with the lineage cocktail, including CD45.1, plus CD44 and CD25 or plus CD4 and CD8. For each time point, the presence of viable DN3, DN4, or DP cells was estimated relative to a fixed number of added microspheres. The ratio at the onset of the culture was set to 100%. The graphs show the mean percentage (± SD) of surviving cells relative to the onset of the culture (100%).
T-cell development in the absence of γ-catenin. (A) Histograms show thymocytes derived from lethally irradiated adult CD45.1+ recipient mice, which were reconstituted with CD45.2+ fetal liver cells of the indicated γ-catenin genotype. Numbers indicate the percentage of CD45.1- (donor-derived) cells. (B) Bar graphs represent the mean (± SD) of the absolute number of CD45.1- total and lin- thymocytes of 5 chimeras. Dot plots show CD45.1- thymocytes stained with CD4 and CD8 (C) or with the lineage cocktail, CD44 and CD25 (D). Numbers indicate the percentage of cells in the respective quadrants. (E) Total thymocytes were incubated for the indicated periods of time in complete culture medium (without the addition of growth factors). After collection, the cells were stained with the lineage cocktail, including CD45.1, plus CD44 and CD25 or plus CD4 and CD8. For each time point, the presence of viable DN3, DN4, or DP cells was estimated relative to a fixed number of added microspheres. The ratio at the onset of the culture was set to 100%. The graphs show the mean percentage (± SD) of surviving cells relative to the onset of the culture (100%).
To address the control of TCF/catenin signaling, we investigated whether pre-TCR signaling controlled β-catenin stability. In the absence of wnt signaling β-catenin is phosphorylated at multiple serine residues in its NH2 terminus, which targets it for proteasomal degradation.18 Wnt signaling prevents the phosphorylation of these serine residues, resulting in the accumulation of active β-catenin, which mediates transcriptional responses upon association with TCF/LEF factors.19 The nonphosphorylated, active form of β-catenin was readily detected in RAG-1-deficient thymocytes (Figure 5C), suggesting that signaling-competent β-catenin is present prior to and arises independent from the pre-TCR.
Next, we induced pre-TCR signaling in RAG-1-deficient mice by injecting anti-CD3ϵ mAb. Thymocytes were harvested prior to CD25 down-regulation and cycling (6 hours) and when a significant fraction of RAG-1-deficient thymocytes are cycling (24 hours).20 Upon the induction of pre-TCR signaling total as well as active β-catenin levels did not increase, strongly suggesting that β-catenin was not stabilized by pre-TCR signaling (Figure 5C). Rather, pre-TCR limited the availability of active β-catenin. Similar to total or active β-catenin, γ-catenin (plakoglobin) also did not increase following pre-TCR signaling (Figure 5C).
Next, we determined TCF-1 levels. In contrast to β-catenin and γ-catenin, the abundance of TCF-1 protein was relatively low in uninduced RAG-1-/- thymocytes. However, TCF-1 levels were considerably increased in anti-CD3ϵ-treated RAG-1-/- thymocytes (Figure 5C). These findings were confirmed using quantitative RT-PCR analysis. As compared with noninjected, Tcf-1 mRNA levels were significantly increased (2.4-fold) at 6 hours and 24 hours after the injection of anti-CD3ϵ mAb (Figure 5D). In contrast, we noted a minor (1.6-fold) and transient (at 6 hours) increase of β-catenin mRNA. Thus, pre-TCR signaling significantly increases TCF-1 expression, which together with the pre-TCR-independent inhibition of β-catenin degradation seems to be necessary for TCF/catenin signaling at the pre-TCR stage.
Discussion
Here, we show that in the absence of TCF-1, pre-TCR-dependent initiation of cycling is dissociated from survival. Because TCF-1 is a nuclear effector of the canonical wnt signaling pathway, this raised the possibility that pre-TCR and wnt signaling had to cooperate to ensure thymocyte survival at the pre-TCR stage. In support of this notion, an increase in TCF/catenin signaling in vivo coincided with pre-TCR signaling. Because the activity of this reporter indicates a high level of wnt/catenin/TCF signaling,12 our data strongly suggest that the nuclear response to wnt signaling depends on the presence of a pre-TCR.
Development of TCF-1-/- thymocytes at the pre-TCR stage was rescued in part by a transgenic TCF-1 containing the NH2-terminal catenin-binding domain. The corresponding domain in TCF-4 and LEF-1 has been shown to bind the wnt signaling intermediates β-catenin and γ-catenin.21 Whereas β-catenin has been shown to bind TCF-1,22 the corresponding information for γ-catenin is not available. Because the importance of β-catenin for thymocyte development has been questioned,10 we have analyzed whether γ-catenin played a role for thymocyte development. Our data show that γ-catenin is not required for thymocyte differentiation in vivo, even though slightly reduced DN survival was observed in vitro. These data together with the analysis of TCF-1 p45 transgenic mice suggest that β-catenin is mediating (at least in part) TCF-1-dependent thymocyte development at the pre-TCR stage. This is indeed consistent with the analysis of independently generated conditional β-catenin knock-out (ko) mice.9 The remaining discrepancy between the phenotypes of β-catenin9 and TCF-1-deficient mice may be due to a redundant function of β- and γ-catenin.
Whereas the phenotype of the β-catenin ko mice9 corresponds to that of TCF-1 ko in part, there are still some differences with respect to its basis. For instance, rather than an effect on survival, Xu et al9 proposed that β-catenin is required for thymocyte cycling. However, similar to the original analysis of TCF-1 ko mice, technical issues may bias the interpretation. For instance, it is difficult to detect apoptotic thymocytes ex vivo by determining the fraction of subdiploid cells. Moreover, preapoptotic, cycling cells are easily lost during lengthy isolation procedures. Thus, it is possible that conditional β-catenin deletion affects thymocyte survival, yet it seems likely that the effect is not as profound as in TCF-1-deficient mice.
Our data suggest a scheme for how the pre-TCR may control wnt signaling. Nonphosphorylated, active β-catenin is present in RAG-1-deficient thymocytes prior to the induction of pre-TCR signaling. Thus β-catenin stabilization in thymocytes occurs independently of the pre-TCR, most likely via the wnt-dependent inhibition of the β-catenin phosphorylation complex. There is indeed evidence that components of the wnt signaling cascade, which are upstream of β-catenin are involved in thymocyte development. Overexpression of antagonists of the wnt receptor Fz/LRP in thymic organ cultures impairs thymocyte expansion.7 Moreover, transgenic overexpression of axin, a negative regulator of wnt signaling, which promotes β-catenin degradation, resulted in widespread thymocyte apoptosis.8 Thus, the available evidence suggests that active, signaling-competent β-catenin is dependent on wnt signals and independent of the pre-TCR.
In contrast to active β-catenin, pre-TCR signaling considerably induced TCF-1 levels. Together with the activation of the wnt reporter at the pre-TCR stage, these findings imply that induction of TCF-1 is the critical pre-TCR-dependent event, which integrates pre-TCR with wnt signals. The importance of regulating TCF-1 levels is also supported by the analysis of p45 TCF-1 Tg mice. The abundance of the Tg p45 protein corresponds approximately to that of the endogenous p45 in total thymocytes.3 However, the amount of the Tg p45 TCF-1 is fixed and not subject to the normal regulation of the endogenous TCF-1 locus. Indeed, the p45 Tg only partially rescued T-cell development at the pre-TCR stage.
Previous work suggested a prosurvival role of β-catenin at the pre-TCR stage.23 The authors deleted the portion of the β-catenin gene, which encodes the phosphorylation sites in the β-catenin protein that determine protein turnover. The accumulation of stable β-catenin induced thymocyte differentiation (without proliferation) and survival past the DN3 stage even in the absence of a pre-TCR. Thus, an excess of stable β-catenin may compensate for the lack of pre-TCR-dependent TCF-1 induction and allow the aberrant survival of pre-TCR-deficient thymocytes. This highlights the need for cells lacking a pre-TCR, to control TCF/catenin signaling to prevent T-cell development past the DN3 stage. The pre-TCR-dependent induction of TCF-1 seems to ensure that only pre-TCR+ thymocytes benefit from the prosurvival function of the wnt pathway.
The TCF-1-dependent effector(s), which ensure thymocyte survival at the pre-TCR stage, remain to be identified. Because survival was rescued by the coactivator-receptive p45 TCF-1 isoform (Figure 4C), we hypothesize that TCF-1 induces the expression of an antiapoptotic effector, such as those of the B-cell lymphoma-2 (Bcl-2) family. Bcl-2 can be excluded because it is normally expressed in the absence of TCF-1,3 and pre-TCR signaling correlates with Bcl-2 down-modulation rather than induction in normal mice (not shown).24 Bcl-xL (B-cell leukemia XL) expression is reduced in TCF-1-deficient thymocytes.3 However, in normal mice significant Bcl-xL expression was not detected prior to the DP stage (not shown). Thus Bcl-xL may be induced too late to ensure the survival of pre-TCR+ thymocytes. Finally, myeloid cell leukemia-1 (Mcl-1) has recently been shown to ensure thymocyte survival at multiple developmental stages, including the pre-TCR stage.25 However, preliminary experiments have failed to demonstrate significant Mcl-1 up-regulation within the first 24 hours after the injection of RAG-1-deficient mice with anti-CD3ϵ mAb (J.C. and W.H., unpublished observation, October 2004). While this manuscript was under review, it was reported that the Bcl-2 family member A1 regulates thymocyte survival at the pre-TCR stage in normal mice.26 Further work will be required to test whether there is a connection between TCF-1 and A1 and/or to identify additional TCF-1-dependent mediator(s) of survival at the pre-TCR stage.
Here we have shown that TCF-1 is required for the survival of cycling pre-TCR+ thymocytes. Genetic evidence suggests that TCF-1 ensures thymocyte survival in part in conjunction with its coactivator β-catenin, which is an intermediate of the canonical wnt signaling pathway. It is further demonstrated that signaling-competent β-catenin arises prior to and thus independent of pre-TCR signaling, most likely via wnt signals. In contrast, pre-TCR signaling induces TCF-1, which coincides with the efficient activation of a wnt reporter gene. Thus, the collaboration of pre-TCR-dependent and pre-TCR-independent signals seems to be necessary to ensure thymocyte survival at the pre-TCR stage.
Prepublished online as Blood First Edition Paper, May 12, 2005; DOI 10.1182/blood-2005-01-0337.
Supported in part by grants from the Swiss National Science Foundation and the Muschamp Foundation (W.H.), the National Center of Competence in Research (NCCR) Molecular Oncology (J.H.), Cancer Research UK (K.W.), Telethon, and Associazione Italiana per la Ricerca sul Cancero (AIRC) (S.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Hans Clevers for TCF-1-deficient mice, Yoshimoto Katsura for anti-TCF-1 antibodies, Anne Wilson and Isabel Ferrero for help with flow cytometry and qPCR, respectively, and Anick Chalifour and Jean-Charles Cerottini for critical reading of the manuscript.

![Figure 1. Thymocyte development in TCF-1-deficient mice. (A) Density plots show a representative analysis of CD4/CD8 and CD44/CD25 subsets among lin- thymocytes (cells not expressing CD4, CD8, CD3, B220, CD11b, TCRβ, TCRγδ, NK1.1, Gr1, and Ter119). Numbers indicate the percentage of cells in the respective gate. (B) Bar graphs show the mean ± SD of the absolute number of cells of the indicated type; DN1 indicates lin- CD44+CD25-/low, DN2, lin- CD44+CD25hi; DN3, lin- CD44-CD25hi; DN4, lin- CD44-CD25neg. Histograms show the expression of CD117 (c-kit) and CD127 (IL-7 receptor α [IL-7Rα]) (C), intracellular TCRβ (D), or CD3ϵ (E) and TCRγδ (F) in the indicated lin- population. Numbers indicate the percentage of cells in the regions defined by horizontal bars. (G) Bar graphs show the mean of the absolute number of cells of the indicated type (± SD).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/5/10.1182_blood-2005-01-0337/6/m_zh80170583290001.jpeg?Expires=1767725469&Signature=zp5S4azezLt~6REajnfWFY9BsXe3wN5ctdwjO1UhkJH5CL6sqLzw7iHIa8Q-nP16cyBnCo1HCqVXpNy8BQskxBUjydDvAW3gPvakhUoRUQQUnqHQBwN24ekqwQxaAx58HewewEgFkp6xLAvUgEQTyuXQgSY3ZkS4dgxxjGfJoyyBIl2FFJSw2SU~oQ4jTBfc7pUd85SaGOdwMq~fuNMaJQks4WLRB22f6GH8TmIkd5itI~Tg23JDbpMDn4TPqe6Ef8ceGEsZM2wyzsYzcJYGX3ibEzvMYCYJgThA1LXQ~RlgtIwC18xohdOmeljsxgUO8T7CFT6ulV10DAwbMb1UEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal