Abstract
Endothelial junctions maintain endothelial integrity and vascular homeostasis. They modulate cell trafficking into tissues, mediate cell-cell contact and regulate endothelial survival and apoptosis. Junctional adhesion molecules such as vascular endothelial (VE)–cadherin and CD31/platelet endothelial cell adhesion molecule (PECAM) mediate contact between adjacent endothelial cells and regulate leukocyte transmigration and angiogenesis. The leukocyte adhesion molecule intercellular adhesion molecule 2 (ICAM-2) is expressed at the endothelial junctions. In this study we demonstrate that endothelial ICAM-2 also mediates angiogenesis. Using ICAM-2–deficient mice and ICAM-2–deficient endothelial cells, we show that the lack of ICAM-2 expression results in impaired angiogenesis both in vitro and in vivo. We show that ICAM-2 supports homophilic interaction, and that this may be involved in tube formation. ICAM-2–deficient cells show defective in vitro migration, as well as increased apoptosis in response to serum deprivation, anti-Fas antibody, or staurosporine. ICAM-2 signaling in human umbilical vein endothelial cells (HUVECs) was found to activate the small guanosine triphosphatase (GTPase) Rac, which is required for endothelial tube formation and migration. These data indicate that ICAM-2 may regulate angiogenesis via several mechanisms including survival, cell migration, and Rac activation. Our findings identify a novel pathway regulating angiogenesis through ICAM-2 and a novel mechanism for Rac activation during angiogenesis.
Introduction
Angiogenesis involves a cascade of events that requires the disassembly of endothelial junctions, followed by detachment, proliferation, and migration of endothelial cells (ECs), and finally the re-establishment of cell-cell and cell-matrix contacts.1 Adhesion molecules at the endothelial junctions, such as CD31/platelet endothelial cell adhesion molecule (PECAM) and junctional adhesion molecule (JAM)–A, support endothelial cell-cell contact through homophilic binding, and are involved in regulating endothelial homeostasis and angiogenesis via different mechanisms. Junctional molecules also mediate leukocyte transendothelial migration through homophilic or heterophilic interactions with leukocytes.2
Intercellular adhesion molecule 2 (ICAM-2) is a member of the immunoglobulin (Ig) superfamily, constitutively expressed at the endothelial junctions.3 Its structure includes 2 extracellular Ig domains, a transmembrane region, and a short intracellular tail.4 ICAM-2, originally identified as a ligand for leukocyte integrin lymphocyte function–associated antigen-1 (LFA-1),4 also interacts with the integrin Mac-15 and the dendritic cell receptor DC-SIGN (dendritic cell–specific ICAM-3–grabbing nonintegrin).6 Through these interactions, ICAM-2 mediates leukocyte trafficking.4,6,7 Recent data indicate that ICAM-2 involvement in leukocyte transmigration may be stimulus specific.8 However its role in inflammation is still unclear: inflammatory cytokines that up-regulate the expression of leukocyte adhesion molecules such as ICAM-1 or vascular cell adhesion molecule 1 (VCAM-1), down-regulate ICAM-2 expression and induce its redistribution away from the junctions.3 A similar pattern is observed for other junctional adhesion molecules such as CD31/PECAM9 and JAM-A.10
On the basis of the similarities in function, structure and localization between ICAM-2 and other junctional adhesion molecules involved in vascular homeostasis, we postulated that ICAM-2 also may play a role in angiogenesis. Data on the regulation of ICAM-2 expression further support this hypothesis. In endothelial cells, ICAM-2 expression can be regulated by cell-cell contact and growth factors (M.-T.H. and A.M.R., manuscript in preparation). ICAM-2 endothelial expression is driven by the transcription factor Erg,11 which is required for endothelial cell (EC) tube formation on Matrigel12 and also regulates the expression of angiogenesis mediators including thrombospondin, SPARC (secreted protein acidic and rich in cysteine), RhoA, and vascular endothelial (VE)–cadherin.12,13
In this paper we demonstrate that ICAM-2 is required for in vitro and in vivo angiogenesis. We also demonstrate for the first time that ICAM-2 can engage in homophilic interaction, and is involved in endothelial survival and migration. Finally, we show that ICAM-2 signaling activates the small guanosine triphosphatase (GTPase) Rac, a key regulator of signal transduction pathways to the cytoskeleton. In conclusion, we have identified a novel angiogenesis pathway, involving the adhesion molecule ICAM-2 and the activation of the small GTPase Rac.
Materials and methods
Animals
ICAM-2–/– mice were generated as described.7 129SV × C57BL/6 wild-type (WT) mice were generated at the Biological Service Unit, Hammersmith Hospital (London, United Kingdom). Experiments were performed according to the Animals Scientific Procedures Act of 1986.
Cells
Murine cardiac endothelial cells (MCECs) were isolated as previously described.14 Briefly, mice heart tissue was diced and incubated with 5 mL of 1 mg/mL collagenase A for 30 minutes at 37°C followed by 5 mL trypsin/EDTA (ethylenediaminetetraacetic acid) for 10 minutes at 37°C. The released ECs were isolated by positive selection with anti-endoglin antibody (Ab; MJ7/18, 10 mg/mL) and MiniMac microbeads system (Miltenyi Biotech, Surrey, United Kingdom). MCECs were cultured on 1% gelatin in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 30 μg/mL endothelial cell growth supplement (ECGS; Sigma, St. Louis, MO), 10 U/mL heparin, 2 mM l-glutamine, 100 IU/mL penicillin, and 0.1 mg/mL streptomycin. Cells were used for experiments at passages 1 and 2. To generate the immortalized endothelial cell lines, passage 1 MCECs from WT or ICAM-2–/– mice were transduced with a retrovirus encoding the temperature-sensitive mutant simian virus 40 (SV40) large T antigen tsTA58, a gift from Prof Yuti Chernajovsky (Barts, London, United Kingdom), as described.15 After infection, the cells were left to recover for 48 hours in fresh medium. Cells were then cultured in the above medium containing G418 (400 μg/mL) at 33°C for 2 to 3 weeks, and resistant colonies were pooled and expanded. Cells were switched to 37°C to arrest proliferation 24 hours before experiments. The endothelial origin of the WT (WT-MCEC-SV)– and ICAM-2–/– (IC2-MCEC-SV)–derived endothelioma lines was verified by fluorescence-activated cell sorting (FACS), using anti-endoglin and anti-CD31 Abs. The phenotype of the IC2-MCEC-SV endothelioma line was similar to that observed in primary MCECs, based on ICAM-2 expression, Matrigel tube formation, and cell proliferation.
HUVECs were isolated as described,16 and cultured in 1% gelatin-coated tissue-culture ware in Medium 199 supplemented with 20% FCS, 30 μg/mL ECGS, 10 U/mL heparin, 2mM l-glutamine, 100 IU/mL penicillin, and 0.1 mg/mL streptomycin.
Flow cytometry
FACS analysis was performed by standard methods.14 Cell samples were incubated with primary or isotype-matched control Abs for 30 minutes at 4°C and washed in phosphate-buffered solution (PBS) twice followed by incubation with fluorescence-conjugated secondary Ab. After 2 more washes in PBS, samples were resuspended in 4% paraformaldehyde and analyzed with Epic XL-MCL flow cytometer and System II software (Beckman Coulter, High Wycombe, United Kingdom). The primary Ab data were normalized against isotype control and expressed as relative fluorescence intensity (RFI).
In vitro Matrigel tube formation assay
Matrigel (300 μL/well) was plated evenly in a 24-well plate, and incubated at 37°C for 30 minutes before adding MCECs (0.5 × 105 cells/well). Tube formation was studied over 8 hours and serial photographs of representative 100 × fields were taken hourly. Endothelial tubes were quantified by counting branches from each EC. Where indicated, cells were preincubated with anti–ICAM-2 or control Ab for 15 minutes at room temperature (RT) before plating onto Matrigel.
In vivo Matrigel plug assay
Matrigel (300 μL) containing 40 ng/mL vascular endothelial growth factor (Peprotech EC, London, United Kingdom) and 10 U/mL heparin (Roche, Lewes, East Sussex, United Kingdom) was injected subcutaneously into the abdominal area of the mouse. Seven days later, Matrigel plugs were carefully excised, fixed with 10% formalin, and processed for hematoxylineosin (H&E) staining. The area of vascular lumen within the plugs was measured by Image ProPlus software (Media Cybernetics, Wokingham, United Kingdom). Two independent experiments were performed, each with 5 to 6 mice per group. For each plug, 5 representative 100 × fields from each of 3 serial sections were analyzed. The data are shown as percentage of vessel-occupying area per 100 × field area.
Endothelial proliferation assay
Cell proliferation was measured over 5 days using the methyl thiazolyl tetrazolium (MTT) assay (Promega, Southampton, United Kingdom). The assay was performed as per manufacturer's instructions, on cells plated onto collagen-coated 96-well tissue-culture plates at 2000 cells/well.
Apoptosis assays
Apoptosis was induced by 3 different methods to investigate both the intrinsic and the extrinsic pathways,17,18 using serum/growth-factor deprivation or staurosporine (20 nM) for the former and anti-Fas Ab for the latter. For the serum deprivation experiments, cells were maintained in 1% bovine serum albumin (BSA)/DMEM for the duration of the experiment. For anti-Fas–induced apoptosis, cells were preincubated with 100 ng/mL interferon-γ (IFN-γ) for 16 hours to up-regulate Fas expression, followed by anti-Fas Ab (10 μg/mL, Clone Jo-2; BD Biosciences, Oxford, United Kingdom) for the indicated time. Apoptosis was evaluated by 2 methods: FACS analysis using the fluorescein isothiocyanate–conjugated annexin V (AnxV)/propidium iodide (PI) assay19 and acridine orange staining of pyknotic nuclei undergoing apoptosis.14
In vitro wound migration assay
The in vitro wound assay was used to measure endothelial unidirectional migration. WT or IC2-MCEC-SV cells were seeded at a density of 6.5 × 105/cm2 onto gelatin-coated 10-mm dishes and allowed to form confluent monolayers. Two separate scratch wounds were generated with a rubber cell scraper (1-mm width). Images were taken at the time of the wound and at intervals up to 48 hours after wounding under phase-contrast microscopy with an Olympus camera (objective, 10 ×; Olympus, London, United Kingdom). Migration was calculated as the distance between the migration front and the wound edge at each time point, as described.20 For each time point, 8 measurements from 4 fields from 2 independent wounds were taken.
Generation of stable ICAM-2 transfectants
Full-length human ICAM-2 in pcDNA3, kindly provided by Dr David Simmons (Oxford, United Kingdom), was used to generate ICAM-2 stable lines. Chinese hamster ovary (CHO) cells were transfected with linearized ICAM-2 plasmid DNA using Lipofectamine 2000 (Invitrogen, Paisley, United Kingdom) as per manufacturer's instructions. Surface ICAM-2 expression on each subclone was analyzed by FACS (anti–ICAM-2 Ab, Clone B-T1; Serotec, Oxford, United Kingdom) and 3 subclones with the highest ICAM-2 expression were selected for further experiments. Mock-transfected cells were generated and grown in parallel. CHO cells stably transfected with E-selectin were generated as described.21
Immunofluorescence
Cells were washed in PBS and fixed in freshly prepared 3% (wt/vol) paraformaldehyde for 20 minutes at room temperature, followed by 50 mM NH4Cl for 10 minutes to neutralize paraformaldehyde. The cells were then processed for indirect immunofluorescence using the anti–human ICAM-2 monoclonal Ab (clone B-T1, 10 μg/mL) followed by a goat anti–mouse Alexa Fluor 555 Ab (Molecular Probes, Eugene, OR) at 10 μg/mL. All incubations were performed at room temperature for 15 minutes in PBS containing 3% BSA. After each incubation, the cells were washed in PBS. Slides were mounted onto coverslips using VectorShield (Vector Laboratories, Peterborough, United Kingdom). Cells were visualized with a Zeiss LSM510-META confocal laser-scanning microscope (Carl Zeiss, Welwyn Garden City, United Kingdom) and images were processed using Adobe Photoshop CS (Adobe Systems, San Jose, CA).
Homophilic ICAM-2 binding assay
CHO-IC2 cells were grown to confluence in 96-well tissue culture plates. A recombinant chimeric molecule with the ICAM-2 extracellular region fused to the Fc portion of human (h) IgG1 (ICAM-2–Fc; R&D Systems, Minneapolis, MN), was preincubated with polystyrene beads conjugated with anti-hFc Ab (HBP-30-5, 1% wt/vol; Spherotech, Libertyville, IL), at 37°C for 1.5 hours. Fifty microliters of the mixture was added to CHO-IC2 cells, to reach a final concentration of 10 μg/mL protein-Fc construct and 0.15% wt/vol beads. After 1.5 hours of incubation at 37°C, unbound beads were carefully removed with 6 washes in warm medium. CHO cells stably transfected with E-selectin (CHO-Esel), ICAM-1–Fc, and VCAM-1–Fc (R&D Systems) were used as control. Experiments were carried out in triplicates. Images from 3 representative 400 × fields from each replicate were taken and bound beads counted. In indicated experiments, anti–ICAM-2 Ab (polyclonal goat anti–ICAM-2 Ab [pAb]; R&D Systems), monoclonal anti–ICAM-2 Ab (mAb), CBR-IC2/2 (Research Diagnostics, Concord, MA) or goat IgG isotype control Ab (R&D Systems) was added to the protein-beads mixture in the final 30 minutes of the preincubation step.
Rac pull-down assay
The ability of Rac-GTP to bind to the p21-binding domain (PBD) of p21-activated kinase (PAK) (PAK1-PBD) was used to study Rac activation.22 To evaluate Rac activation during MCEC tube formation on Matrigel, cell extracts were prepared at 40 minutes and 4 hours after plating on Matrigel. For the ICAM-2 cross-linking experiments, HUVECs were grown in full medium until 80% to 90% confluent, and subsequently maintained in 1% BSA/M199 for 16 hours. Cells were then incubated with pAb (15 μg/mL) for 40 minutes at 37°C, followed by incubation with secondary cross-linking Ab (30 μg/mL) for the indicated time. Control cells were treated with primary or secondary Ab alone. For total cell extracts, cells were washed twice with Hanks balanced salt solution (HBSS) and lysed with ice-cold buffer containing phosphatase and protease inhibitors. After sonication for 30 seconds and centrifugation at 14 000g for 5 minutes at 4°C, the supernatants were incubated with glutathione S-transferase (GST)–PBD agarose beads (Upstate Biotechnology, Dundee, United Kingdom) for 1 hour at 4°C. The bound fraction was separated onto a sodium dodecyl sulfate (SDS)–polyacrylamide gel and the presence of Rac in the bound fraction was detected by Western blotting using primary anti-Rac Ab (clone 23A8; Upstate Biotechnology). The amount of activated Rac was normalized against total Rac in the corresponding lysate. Integrated density values of the bands were measured using Alpha Innotech Chemilmager 5500 program (Alpha Innotech, San Leandro, CA).
Results
Endothelial ICAM-2 is required for in vitro angiogenesis
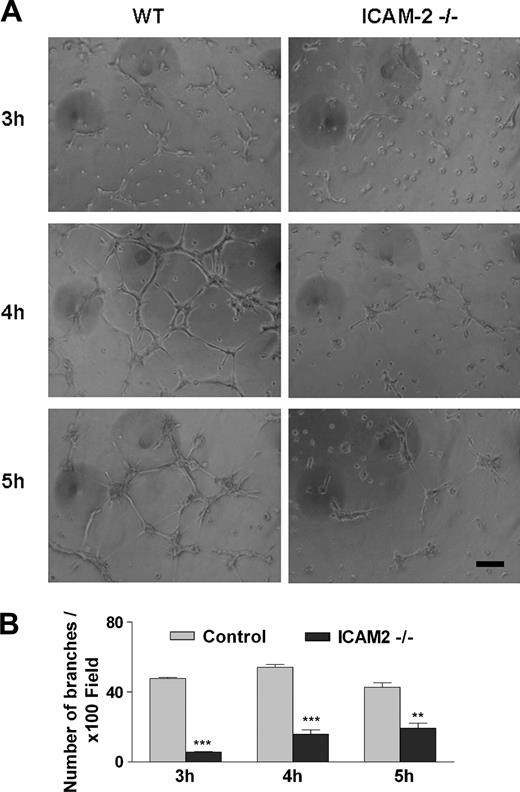
To determine whether ICAM-2 is involved in angiogenesis, we studied the ability of primary ICAM-2–deficient ECs to form tubes in an in vitro tube formation assay on Matrigel. MCECs were isolated from mouse hearts by positive endoglin selection. The endothelial origin of MCECs was confirmed by FACS analysis of endoglin and CD31/PECAM-1 expression; the lack of ICAM-2 expression in the ICAM-2–/– cells was also confirmed (not shown). MCECs from ICAM-2–/– or WT mice were seeded on Matrigel and images of representative fields were taken hourly. In WT MCEC samples, network formation took place between 3 and 6 hours after seeding. Tube formation was quantified after 3, 4, and 5 hours by counting branches from 3 representative fields per replicate. As shown in Figure 1A, ICAM-2–/– cells showed a significantly impaired ability to form tubes on Matrigel compared with WT MCECs. A significant reduction in tube formation in the ICAM-2–/– cells compared with WT was observed at all time points studied (Figure 1B). The difference was most marked at 3 and 4 hours, when a tubular network of interconnecting branches was well established in WT samples, while few contacts between cells with shorter protrusions and few branches were observed in the ICAM-2–/– samples. These data indicate that ICAM-2 is required for endothelial tube formation on Matrigel.
ICAM-2 mediates angiogenesis in vitro: tube formation on Matrigel. (A) Primary murine cardiac endothelial cells (MCECs) isolated from control (WT) or ICAM-2–/– mice were seeded on Matrigel. In each experiment, 6 age- and sex-matched mice/group were used. The figure shows 1 representative of 3 separate experiments, each performed in triplicate. Left panels show WT; right panels, ICAM-2–/–. In WT, cells were spreading, elongating, and making contacts with neighboring cells at 3 hours, and an interconnecting tube network was formed over 4 to 5 hours. At all time points studied, the branches and tubes formed by ICAM-2–/– cells were much less developed compared with control. Scale bar equals 100 μm. Images were acquired using a Leitz Labovert inverted microscope (Leica Microsystems, Milton Keynes, United Kingdom) fitted with a 10 × phase-contrast objective lens (Leitz-Phaco 10 ×/0.25 NA). Images were captured with a digital camera model DP50-CU (Olympus) using Viewfinder Lite (v. 1) software (Olympus). Image processing was carried out using Adobe Photoshop CS (Adobe Systems). (B) Branches from each cell were counted from 3 representative 100 × field/well. Error bars indicate mean ± SEM. **P < .01; ***P < .001 analysis of variance (ANOVA).
ICAM-2 mediates angiogenesis in vitro: tube formation on Matrigel. (A) Primary murine cardiac endothelial cells (MCECs) isolated from control (WT) or ICAM-2–/– mice were seeded on Matrigel. In each experiment, 6 age- and sex-matched mice/group were used. The figure shows 1 representative of 3 separate experiments, each performed in triplicate. Left panels show WT; right panels, ICAM-2–/–. In WT, cells were spreading, elongating, and making contacts with neighboring cells at 3 hours, and an interconnecting tube network was formed over 4 to 5 hours. At all time points studied, the branches and tubes formed by ICAM-2–/– cells were much less developed compared with control. Scale bar equals 100 μm. Images were acquired using a Leitz Labovert inverted microscope (Leica Microsystems, Milton Keynes, United Kingdom) fitted with a 10 × phase-contrast objective lens (Leitz-Phaco 10 ×/0.25 NA). Images were captured with a digital camera model DP50-CU (Olympus) using Viewfinder Lite (v. 1) software (Olympus). Image processing was carried out using Adobe Photoshop CS (Adobe Systems). (B) Branches from each cell were counted from 3 representative 100 × field/well. Error bars indicate mean ± SEM. **P < .01; ***P < .001 analysis of variance (ANOVA).
Endothelial ICAM-2 is required for in vivo angiogenesis
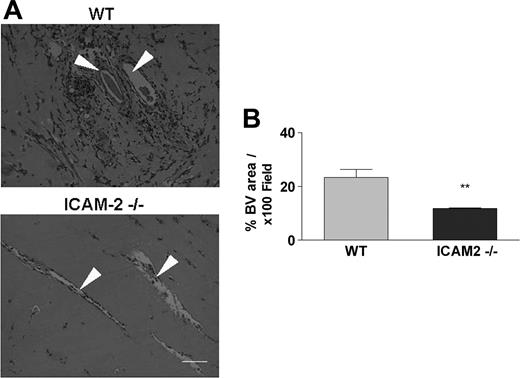
To validate the defect observed in vitro, we studied angiogenesis in vivo in using the Matrigel plug assay.23 Matrigel was injected subcutaneously into the abdominal areas of WT and ICAM-2–/– mice; after 7 days, the vascularized plugs were removed and processed for H&E staining to identify the area covered by vessels. As shown in Figure 2A, new vessel formation within the plugs, accompanied by erythrocyte and leukocyte infiltration, was observed in plug samples from WT mice (top). Samples from ICAM-2–/– mice (bottom) showed significantly less vascular and cellular infiltration within the plugs. Measurement of the neovascularized area showed a 50% reduction in the vessel-containing area in the ICAM-2–/– mice compared with WT mice (32% ± 5.99% vs 11.8% ± 0.42%; Figure 2B).23 Therefore, both in vitro and in vivo assays demonstrate defective angiogenesis in the ICAM-2–/– mice.
ICAM-2 mediates angiogenesis in vivo: Matrigel plug assay. Matrigel plugs were generated by subcutaneous injection of Matrigel; the plugs were removed 7 days later and processed for H&E staining. (A) In samples from WT mice (top), formation of vascular structures within the plugs is accompanied by erythrocyte and leukocyte infiltration. In samples from ICAM-2–/– mice (bottom) a significant reduction in vascular structures and cellular infiltration within the plugs was observed. Arrowheads show blood vessels. Scale bar equals 100 μm. Images were acquired as in Figure 1A. (B) The vascular lumen area was measured using Image ProPlus software. The average vessel-containing area was calculated from 5 representative 100 × fields of 3 representative serial sections of each plug and is shown as the percentage of area occupied by blood vessel (BV)/100 × field. Compared with WT mice, a 50% reduction in vessel-containing areas were observed in samples from ICAM-2–/– mice. Data represent 1 of 2 independent experiments; 5 to 6 mice per group were used in each experiment. Error bars indicate mean ± SEM. **P < .01, unpaired t test.
ICAM-2 mediates angiogenesis in vivo: Matrigel plug assay. Matrigel plugs were generated by subcutaneous injection of Matrigel; the plugs were removed 7 days later and processed for H&E staining. (A) In samples from WT mice (top), formation of vascular structures within the plugs is accompanied by erythrocyte and leukocyte infiltration. In samples from ICAM-2–/– mice (bottom) a significant reduction in vascular structures and cellular infiltration within the plugs was observed. Arrowheads show blood vessels. Scale bar equals 100 μm. Images were acquired as in Figure 1A. (B) The vascular lumen area was measured using Image ProPlus software. The average vessel-containing area was calculated from 5 representative 100 × fields of 3 representative serial sections of each plug and is shown as the percentage of area occupied by blood vessel (BV)/100 × field. Compared with WT mice, a 50% reduction in vessel-containing areas were observed in samples from ICAM-2–/– mice. Data represent 1 of 2 independent experiments; 5 to 6 mice per group were used in each experiment. Error bars indicate mean ± SEM. **P < .01, unpaired t test.
ICAM-2 supports homophilic interaction
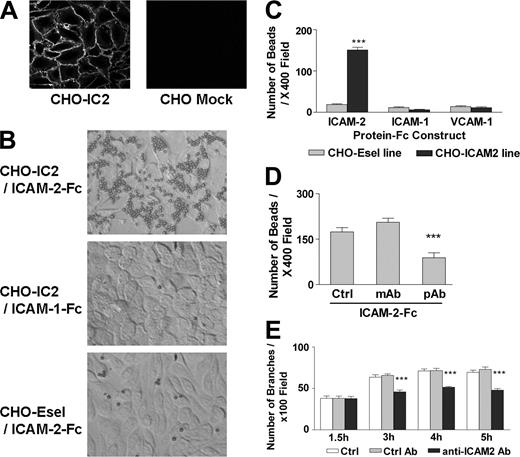
Having demonstrated that endothelial ICAM-2 is involved in angiogenesis, we investigated the underlying mechanism. The assembly and disassembly of endothelial junctions is critical in the regulation of angiogenesis. Other junctional molecules, such as CD31/PECAM and JAM-A, engage in homophilic interaction between adjacent EC.24,25 Because of its similarities to these molecules, we speculated that ICAM-2 could also engage in homophilic binding. To test this hypothesis, stable CHO cell lines expressing ICAM-2 (CHO-IC2) were generated for ligand-binding studies. Surface ICAM-2 expression in these clones was verified by FACS (not shown) and immunofluorescent staining, which showed that ICAM-2 expression was concentrated mainly at the cell junctions (Figure 3A). The recombinant soluble chimeric protein ICAM-2–Fc, coupled to anti-Fc–conjugated polystyrene beads, was used as ligand. CHO cells stably expressing E-selectin (CHO-Esel) and bead-coupled recombinant chimeric molecules ICAM-1–Fc and VCAM-1–Fc were used as controls. Figure 3B shows that ICAM-2–Fc bound to CHO-IC2 (top) but not CHO-Esel cells (bottom), indicating that ICAM-2 supports homophilic interaction. The interaction was specific to ICAM-2, since neither ICAM-1–Fc beads (Figure 3B, middle) nor VCAM-1–Fc beads (not shown) bound to CHO-IC2 cells. Quantification of bound protein-coupled beads to CHO-IC2 or CHO-Esel is shown in Figure 3C. Only ICAM-2–Fc–coupled beads, but not ICAM-1–Fc– or VCAM-1–Fc–coupled beads, bound to CHO-IC2 cells. No binding to CHO-Esel was detected with any of the recombinant chimeric proteins. ICAM-2–Fc also bound to ICAM-2 on resting HUVECs (data not shown). Therefore ICAM-2, like other endothelial junctional molecules, can engage in homophilic interaction.
To determine whether ICAM-2 homophilic interaction was required for endothelial tube formation, we first searched for an antibody that could inhibit this interaction. Two anti–ICAM-2 antibodies were screened in the bead binding assay. ICAM-2–Fc binding to CHO-IC2 cells was specifically inhibited by a pAb anti–ICAM-2 antibody but not by the mAb CBR/IC2-2 or control Ab (Figure 3D). Interestingly, this mAb is known to inhibit ICAM-2/LFA-1 interaction,26 suggesting that the epitope(s) involved in ICAM-2 homophilic interaction may be different from those involved in binding to integrins. We then addressed the question whether ICAM-2 homophilic interaction is required for angiogenesis, by testing the effect of the anti–ICAM-2 pAb which inhibits ICAM-2 homophilic interaction (Figure 3D) on in vitro endothelial tube formation on Matrigel. In the presence of the ICAM-2 pAb, tube formation on Matrigel was significantly inhibited by 30% compared with untreated control samples, or with control Ab (Figure 3E). These results suggest that ICAM-2 homophilic interaction may be involved in endothelial tube formation and angiogenesis.
ICAM-2 supports homophilic interaction. (A) ICAM-2 surface expression on CHO cells stably transfected with human ICAM-2 (CHO-IC2), detected by immunofluorescence using an anti–ICAM-2 mAb (clone B-T1). ICAM-2 expression is concentrated at the cell junctions, similarly to what is observed in endothelial cells. Staining of mock-transfected CHO cells with the same antibody is also shown. (B) ICAM-2 homophilic interaction. Polystyrene beads conjugated with anti–human Fc Ab, preincubated with soluble ICAM-2–Fc, were added to CHO-IC2 (top) or control cells (CHO-Esel, bottom) in 96-well plates. ICAM-1–Fc (middle) or VCAM-1–Fc (image not shown) were used as control proteins. Images were acquired as in Figure 1A. (C) The number of beads retained in the wells after washings was counted per 400 × field of each replicate. Experiments were performed in triplicate. ICAM-2–Fc significantly bound to CHO-IC2 cells, compared with ICAM-1–Fc and VCAM-1–Fc. None of the protein-Fc constructs bound to CHO-Esel. ***P < .001, compared with control protein constructs and binding to CHO-Esel, ANOVA. n = 4. (D) Two anti–ICAM-2 Abs were tested for their ability to block ICAM-2 homophilic interaction, by preincubation with the protein-Fc-beads complex before adding to CHO-IC2. The polyclonal anti–ICAM-2 Ab (pAb), but not the monoclonal anti–ICAM-2 Ab (CBR-IC2/2) or goat IgG isotype control (Ctrl), significantly inhibited binding of ICAM-2 Fc to CHO-IC2 by 50%. ***P < .001, ANOVA. n = 3. (E) Matrigel tube formation is inhibited by anti–ICAM-2 mAb. HUVECs were preincubated with pAb anti–ICAM-2 or goat IgG control for 15 minutes before plating onto Matrigel. Photos were taken hourly after seeding, and branches counted as described (see “Materials and methods”). In the presence of anti–ICAM-2 pAb, HUVEC tube formation was inhibited by approximately 30% compared with control Ab (Ctrl Ab) or untreated samples (Ctrl) at 3, 4, and 5 hours. ***P < .001, ANOVA. n = 3. (C-E) Error bars indicate mean ± SEM.
ICAM-2 supports homophilic interaction. (A) ICAM-2 surface expression on CHO cells stably transfected with human ICAM-2 (CHO-IC2), detected by immunofluorescence using an anti–ICAM-2 mAb (clone B-T1). ICAM-2 expression is concentrated at the cell junctions, similarly to what is observed in endothelial cells. Staining of mock-transfected CHO cells with the same antibody is also shown. (B) ICAM-2 homophilic interaction. Polystyrene beads conjugated with anti–human Fc Ab, preincubated with soluble ICAM-2–Fc, were added to CHO-IC2 (top) or control cells (CHO-Esel, bottom) in 96-well plates. ICAM-1–Fc (middle) or VCAM-1–Fc (image not shown) were used as control proteins. Images were acquired as in Figure 1A. (C) The number of beads retained in the wells after washings was counted per 400 × field of each replicate. Experiments were performed in triplicate. ICAM-2–Fc significantly bound to CHO-IC2 cells, compared with ICAM-1–Fc and VCAM-1–Fc. None of the protein-Fc constructs bound to CHO-Esel. ***P < .001, compared with control protein constructs and binding to CHO-Esel, ANOVA. n = 4. (D) Two anti–ICAM-2 Abs were tested for their ability to block ICAM-2 homophilic interaction, by preincubation with the protein-Fc-beads complex before adding to CHO-IC2. The polyclonal anti–ICAM-2 Ab (pAb), but not the monoclonal anti–ICAM-2 Ab (CBR-IC2/2) or goat IgG isotype control (Ctrl), significantly inhibited binding of ICAM-2 Fc to CHO-IC2 by 50%. ***P < .001, ANOVA. n = 3. (E) Matrigel tube formation is inhibited by anti–ICAM-2 mAb. HUVECs were preincubated with pAb anti–ICAM-2 or goat IgG control for 15 minutes before plating onto Matrigel. Photos were taken hourly after seeding, and branches counted as described (see “Materials and methods”). In the presence of anti–ICAM-2 pAb, HUVEC tube formation was inhibited by approximately 30% compared with control Ab (Ctrl Ab) or untreated samples (Ctrl) at 3, 4, and 5 hours. ***P < .001, ANOVA. n = 3. (C-E) Error bars indicate mean ± SEM.
ICAM-2 protects endothelial cells from apoptosis. (A) Serum starvation-induced apoptosis. Cells were maintained in DMEM with 1% BSA for 24 or 48 hours. Apoptosis was quantified by FACS analysis using the annexin V (AnxV) and propidium iodide (PI) method (A, inset) and shown as fold increase of the AnxV (+)/PI (–) cells compared with control. (B) Anti-Fas–induced apoptosis. MCECs were treated with 100 ng/mL IFN-γ for 16 hours followed by 10 μg/mL anti-Fas Ab for 24 or 48 hours. Data are shown as fold increase of the AnxV (+)/PI (–) cells compared with control. (C) Staurosporine-induced apoptosis. MCECs were incubated with 20 nM staurosporine for 3 or 6 hours. Apoptosis was measured by acridine orange staining (C, inset) and pyknotic nuclei count. Image was acquired as in Figure 1A. Data are shown as average percentage of apoptotic cells/200 × field. Scale bar equals 20 μm. With all 3 stimuli, MCECs from ICAM-2–/– mice were significantly more susceptible to apoptosis compared with WT MCECs. *Comparison with WT cells at the same time point. †Comparison with ICAM-2–/– cells at 24 hours. *P < .05; ***P < .001; †P < 0.05, ‡P < .01, ANOVA. n = 3. (A-C) Error bars indicate mean ± SEM.
ICAM-2 protects endothelial cells from apoptosis. (A) Serum starvation-induced apoptosis. Cells were maintained in DMEM with 1% BSA for 24 or 48 hours. Apoptosis was quantified by FACS analysis using the annexin V (AnxV) and propidium iodide (PI) method (A, inset) and shown as fold increase of the AnxV (+)/PI (–) cells compared with control. (B) Anti-Fas–induced apoptosis. MCECs were treated with 100 ng/mL IFN-γ for 16 hours followed by 10 μg/mL anti-Fas Ab for 24 or 48 hours. Data are shown as fold increase of the AnxV (+)/PI (–) cells compared with control. (C) Staurosporine-induced apoptosis. MCECs were incubated with 20 nM staurosporine for 3 or 6 hours. Apoptosis was measured by acridine orange staining (C, inset) and pyknotic nuclei count. Image was acquired as in Figure 1A. Data are shown as average percentage of apoptotic cells/200 × field. Scale bar equals 20 μm. With all 3 stimuli, MCECs from ICAM-2–/– mice were significantly more susceptible to apoptosis compared with WT MCECs. *Comparison with WT cells at the same time point. †Comparison with ICAM-2–/– cells at 24 hours. *P < .05; ***P < .001; †P < 0.05, ‡P < .01, ANOVA. n = 3. (A-C) Error bars indicate mean ± SEM.
ICAM-2 protects endothelial cells from apoptosis
Apoptosis is an important regulator of angiogenesis1 and junctional adhesion molecules can provide survival signals to ECs. ICAM-2 has been shown to protect T cells and ICAM-2–overexpressing fibroblasts from apoptosis.27 Therefore, we speculated that ICAM-2 may support angiogenesis in part by providing survival signals to ECs. To test this hypothesis, the ability of WT and ICAM-2–/– MCECs to withstand apoptotic stimulation was investigated. Apoptosis was induced in primary MCECs isolated from ICAM-2–/– or WT mice by serum and growth-factor deprivation (Figure 4A), anti-Fas Ab (Figure 4B), or staurosporine (Figure 4C). Cell death was measured by Anx-V/PI staining (Figure 4A, inset) or by acridine orange staining (Figure 4C, inset). With all stimuli used, ICAM-2–/– cells were significantly more prone to apoptosis than WT cells. Serum and growth-factor deprivation induced a significant increase in apoptosis in the ICAM-2–/– cells after 48 hours, compared with WT (Figure 4A). A similar pattern was observed when apoptosis was induced by anti-Fas antibody (Figure 4B), with significantly increased apoptosis in the ICAM2–/– cells after 48 hours of stimulation, compared with WT cells. Staurosporine-induced apoptosis was significantly higher in the ICAM-2–/– cells compared with WT cells at both 3 hours and 6 hours after stimulation (Figure 4C). These data indicate that the lack of ICAM-2 expression predisposes endothelial cells to apoptosis and that ICAM-2 provides protection against both intrinsic and extrinsic pathways of apoptosis. The susceptibility of the ICAM-2–/– cells to apoptosis may, at least in part, be responsible for the defect in angiogenesis observed in the ICAM-2–/– mice.
ICAM-2 is not involved in endothelial proliferation
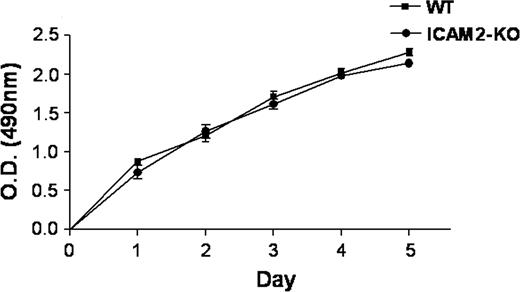
Another possible mechanism by which ICAM-2 may be involved in angiogenesis is through regulation of endothelial cell proliferation. To test this hypothesis, proliferation of primary MCECs from ICAM-2–/– or WT mice was measured in vitro using the MTT assay over 5 days. As shown in Figure 5, no difference in proliferation over 5 days was observed between ICAM-2–/– and WT cells, suggesting that the defect in angiogenesis exhibited by the ICAM-2–/– mice is independent of endothelial cell proliferation.
ICAM-2 is involved in endothelial-cell migration
Endothelial migration is a key step in angiogenesis, and involves major reorganization of the actin cytoskeleton. ICAM-2 is linked to the actin cytoskeleton via the ezrin-radixin-moesin (ERM) family, which acts as linkers between plasma membrane proteins and the cytoskeleton,28 and has been shown to regulate cell migration.29 We speculated that the absence of ICAM-2 could affect endothelial migration. In order to test this hypothesis, unidirectional migration of endothelial cells was assessed using an in vitro wound-healing assay. Confluent monolayers of MCEC-SV from WT or ICAM-2–/– mice were wounded using a thick rubber scraper. The average width of the wound was similar in WT-MCEC-SV compared with IC2-MCEC-SV monolayers (mean ± SD: 897.7 μm ± 188.7 μm vs 971.4 μm ± 115 μm, respectively; P = .645). Pictures of the wound were taken over 48 hours. ICAM2–/– MCEC-SV migrated more slowly than WT cells; the difference in migration rate was significant at 14, 18, 22.5, and 23.5 hours (Figure 6). At 40 hours, however, both WT and ICAM2–/– cells filled the wound area (not shown). These results indicate that ICAM-2 is involved in EC migration, and this is likely to contribute to the angiogenesis defect observed in the ICAM-2–/– mice.
ICAM-2 regulates Rac activation during endothelial-tube formation
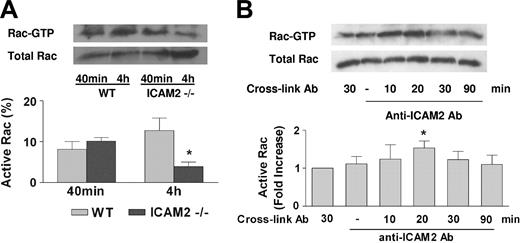
The defect in cell migration observed in the ICAM-2–deficient cells suggests that ICAM-2 may be required to trigger signal transduction pathways involved in migration. Rho-like GTPases are key regulators of the cytoskeletal changes required for cell migration. We therefore speculated that ICAM-2 may regulate small GTPases and mediate angiogenesis through this mechanism. Of the best-characterized small GTPases, Rac was shown to regulate capillary tube formation on Matrigel.30 Therefore, we tested whether ICAM-2 is required for Rac activation during tube formation on Matrigel. Rac activity in MCECs from WT or ICAM2–/– mice seeded on Matrigel was measured by GST-PBD pull-down assay.22 As shown in Figure 7A, Rac was activated in WT cells during spreading (40 minutes), and the activation was sustained at 4 hours, coinciding with maximal tubular network formation. In the ICAM-2–/– cells, Rac activation at the spreading stage (40 minutes) was similar to WT. However ICAM-2–/– cells failed to sustain Rac activation at 4 hours and formed significantly reduced tubular networks. These results show that ICAM-2 is required to sustain Rac activation during tube formation.
ICAM-2 does not affect endothelial cell proliferation. Cell proliferation was measured over 5 days on WT and ICAM-2–/– MCECs plated onto collagen-coated 96-well tissue culture using the MTT assay. No difference in the proliferation rate of MCECs was observed in the ICAM-2–deficient cells. Data are presented as absorbance at optical density (OD) 490 nm (SEM ± SD of 3 replicate experiments).
ICAM-2 does not affect endothelial cell proliferation. Cell proliferation was measured over 5 days on WT and ICAM-2–/– MCECs plated onto collagen-coated 96-well tissue culture using the MTT assay. No difference in the proliferation rate of MCECs was observed in the ICAM-2–deficient cells. Data are presented as absorbance at optical density (OD) 490 nm (SEM ± SD of 3 replicate experiments).
ICAM-2 is involved in endothelial-cell migration. WT-MCEC-SV and IC2-MCEC-SV cells were used in a wound-healing assay. Confluent monolayers were scratch-wounded and incubated for up to 48 hours. (A) Photographs of wounded monolayers taken at 1 and 23.5 hours. The vertical lines indicate the wound edge and the migration front (dotted line). The horizontal arrows indicate the migration distance. Scale bar equals 50 μm. Images were acquired as in Figure 1A. (B) Migration distance, measured at 14, 18, 22.5, and 23.5 hours. IC2-MCEC-SVs show decreased migration at all time points, compared with WT. Values represent mean ± SD of 8 measurements. *P < .001, unpaired t test.
ICAM-2 is involved in endothelial-cell migration. WT-MCEC-SV and IC2-MCEC-SV cells were used in a wound-healing assay. Confluent monolayers were scratch-wounded and incubated for up to 48 hours. (A) Photographs of wounded monolayers taken at 1 and 23.5 hours. The vertical lines indicate the wound edge and the migration front (dotted line). The horizontal arrows indicate the migration distance. Scale bar equals 50 μm. Images were acquired as in Figure 1A. (B) Migration distance, measured at 14, 18, 22.5, and 23.5 hours. IC2-MCEC-SVs show decreased migration at all time points, compared with WT. Values represent mean ± SD of 8 measurements. *P < .001, unpaired t test.
To investigate further the relationship between ICAM-2 signaling and Rac activation, Rac activation in HUVECs following cross-linking of ICAM-2 with the anti–ICAM-2 pAb, which blocks ICAM-2 homophilic interaction and HUVEC tube formation on Matrigel, was investigated. As shown in Figure 7B, ICAM-2 cross-linking resulted in Rac activation, with a peak at 20 minutes after cross-linking and a return to baseline after 30 minutes. These results indicate that ICAM-2 homophilic interaction directly activates Rac and identifies a novel pathway for Rac activation during endothelial junction formation and angiogenesis.
ICAM-2 regulates activation of the small GTPase Rac during endothelial tube formation. (A) Rac activation during Matrigel tube formation. MCECs plated onto Matrigel were analyzed for Rac activation at spreading (40 minutes) and tube formation stage (4 hours) by GST-PBD pull-down assays. During spreading (40 minutes), ICAM-2–/– and WT cells had comparable levels of Rac activation; however, during tube formation (4 hours), ICAM-2–/– cells were unable to sustain Rac activation. *P < .05, unpaired t test. n = 4. (B) ICAM-2 signaling induces Rac activation. HUVECs grown to 80% confluence were starved for 16 hours and incubated with goat anti–ICAM-2 Ab at 15 μg/mL (pAb) for 30 minutes, followed by cross-linking with anti–goat IgG Ab. Rac activation was measured at 10, 20, 30, and 90 minutes following cross-linking. Negative control samples were incubated with either the cross-linking Ab or primary Ab alone. ICAM-2 cross-linking induced Rac activation, which peaked at 20 minutes and returned to baseline after 30 minutes. *P < .05, unpaired t test. n = 5. (A-B) Error bars indicate mean ± SEM.
ICAM-2 regulates activation of the small GTPase Rac during endothelial tube formation. (A) Rac activation during Matrigel tube formation. MCECs plated onto Matrigel were analyzed for Rac activation at spreading (40 minutes) and tube formation stage (4 hours) by GST-PBD pull-down assays. During spreading (40 minutes), ICAM-2–/– and WT cells had comparable levels of Rac activation; however, during tube formation (4 hours), ICAM-2–/– cells were unable to sustain Rac activation. *P < .05, unpaired t test. n = 4. (B) ICAM-2 signaling induces Rac activation. HUVECs grown to 80% confluence were starved for 16 hours and incubated with goat anti–ICAM-2 Ab at 15 μg/mL (pAb) for 30 minutes, followed by cross-linking with anti–goat IgG Ab. Rac activation was measured at 10, 20, 30, and 90 minutes following cross-linking. Negative control samples were incubated with either the cross-linking Ab or primary Ab alone. ICAM-2 cross-linking induced Rac activation, which peaked at 20 minutes and returned to baseline after 30 minutes. *P < .05, unpaired t test. n = 5. (A-B) Error bars indicate mean ± SEM.
Discussion
Endothelial junctions govern vascular homeostasis by regulating leukocyte transmigration, permeability, endothelial cell survival, and proliferation. Adhesion molecules expressed at the endothelial junctions mediate contact with leukocytes and with neighboring endothelial cells. Ligand binding, both homophilic and heterophilic, results in the activation of signal transduction pathways. Examples are CD31/PECAM, which is involved in regulating both leukocyte trafficking into tissue and endothelial survival,31 and JAM-A, which has been shown to regulate monocyte migration32 as well as endothelial cell motility.33 Therefore, it appears that leukocyte adhesion molecules play multiple roles in endothelial cells.
ICAM-2 is concentrated at endothelial cell-cell contacts3 and is known as a leukocyte adhesion molecule. In this study we demonstrate that ICAM-2 can also regulate angiogenesis, and identify several new functions for ICAM-2 which are related to its role in angiogenesis. Using ICAM-2–/– mice and primary endothelial cells derived from these mice, we show that lack of ICAM-2 expression on ECs results in defective angiogenesis, both in vitro and in vivo. A role for ICAM-2 in angiogenesis in vivo is further supported by data from Melero et al, which showed that an anti–ICAM-2 Ab eradicated established murine colon carcinoma through mechanisms involving cytotoxic T-cell activation and, to a lesser extent, inhibition of angiogenesis.34 In our study, defective angiogenesis in the ICAM-2–/– mouse in vivo was reflected in the inability of ICAM-2–deficient endothelial cells to form tubes on Matrigel in vitro, indicating a primary endothelial defect.
Several studies have shown that ICAM-2 expression on endothelial cells is concentrated at the junctions, although the exact location of ICAM-2 within junctions has not yet been determined. Adhesion between adjacent endothelial cells is mediated by homophilic interactions between junctional molecules, forming a zipper-like structure.35 We therefore investigated whether ICAM-2 could support homophilic interaction. Using CHO transfectants stably overexpressing ICAM-2, we showed that ICAM-2 is capable of supporting homophilic interaction. This is the first evidence of the presence of an endothelial ligand for ICAM-2, suggesting that ICAM-2 may contribute to the formation of junctional structures. Tube formation on Matrigel was inhibited by an anti–ICAM-2 antibody that also blocked homophilic interaction, suggesting that the homophilic interaction between ICAM-2 on opposing ECs may be involved in angiogenesis. Further experiments with purified F(ab)′ fragments and recombinant ICAM-2–Fc will be required to define the role of ICAM-2 homophilic interaction in angiogenesis. Our results do not rule out the possibility that ICAM-2 may interact with other endothelial ligands, or that ICAM-2 may engage with ICAM-2 on leukocytes or platelets.
Several mechanisms could be responsible for the angiogenesis defect in the ICAM-2–/– mice. Cross-linking of ICAM-2 has been shown to protect a variety of cells, including T/B lymphocytic cell lines, primary human B cells, and ICAM-2–overexpressing fibroblasts, from apoptosis27 ; however, no data are available on the role of ICAM-2 in endothelial apoptosis. Inhibition of EC apoptosis is an essential step in angiogenesis, and the induction of apoptosis counteracts angiogenesis.36,37 In this paper we show that ICAM-2–deficient endothelial cells are more susceptible to apoptosis. The lack of ICAM-2–dependent survival signals on the ICAM-2–deficient ECs could therefore contribute to the defect in angiogenesis shown here. The ICAM-2–dependent antiapoptotic effects described in other cell types were shown to be linked to the phosphatidylinositol 3-kinase (PI-3K)/Akt pathway,27 which plays a critical role in regulating EC survival, cell migration, and tube formation.38 Studies are underway to determine whether this pathway is involved in ICAM-2–mediated survival signals in ECs.
Angiogenesis involves endothelial cell spreading, migration, and regulation of cell-cell and cell-matrix adhesion,39 all of which require significant reorganization of the actin cytoskeleton. The cytoplasmic tail of ICAM-2 is linked to the actin cytoskeleton by the ezrin-radixin-moesin (ERM) family, which acts as linkers between plasma membrane proteins and the cytoskeleton.28 The absence of ICAM-2 could alter ERM distribution and/or function and, as a consequence, the actin cytoskeleton itself, hence affecting cell shape change, migration, and junction formation. We found no difference in ezrin distribution between ICAM-2–deficient ECs and control ECs (M.-T.H. and A.M.R., unpublished observation, September 2004). However, the distribution of other members of the ERM family, or indeed their activation state, was not investigated; therefore, it is possible that a defect in ERM function in these cells may exist. ERM controls cell adhesion and migration through the Rho family of small GTPases,40 which play a critical role in regulating cytoskeletal dynamics, cell migration, and junction formation.41 We have previously shown that cross-linking of ICAM-2 on HUVECs does not activate RhoA.16 The small GTPase Rac has been shown to be required for tube formation on Matrigel.30 In this study, we demonstrate that ICAM-2 cross-linking activates Rac in HUVECs, and that endothelial ICAM-2 is required for Rac activation during tube formation. Although during spreading Rac activation was similar in ICAM-2–deficient cells and WT cells, ICAM-2–deficient cells failed to sustain Rac activation during formation of the tube network. These results indicate that ICAM-2 is required to maintain Rac activation during tube formation. The defect in Rac activation could also explain the delayed endothelial cell migration of ICAM-2–/– cells observed in the in vitro wound-healing assay, compared with WT. It is worth noting that ICAM-2 expression in the WT-MCEC-SV line was low; hence, other ICAM-2–independent mechanisms contributing to this phenotype cannot be ruled out. Our findings suggest that ICAM-2–dependent activation of Rac results in defective endothelial cell migration. ICAM-2 therefore may regulate angiogenesis at least in part via its ability to activate Rac at the migrating/branching stage.
Rac plays multiple roles in endothelial cells; therefore, the finding that ICAM-2 regulates Rac suggests that other mechanisms may also contribute to the overall defect in angiogenesis observed in the ICAM-2–deficient mice. Rac regulates endothelial junction assembly and function as well as cadherin adhesiveness.42 VE-cadherin itself can regulate Rac activation via the guanosine exchange factor Tiam,43 indicating a bidirectional cross-talk between junctional signaling molecules and small GTPases of the Rho family. Our results suggest that during the establishment of endothelial junctions Rac activation requires at least 2 distinct junctional signaling events, one VE-cadherin–dependent and another ICAM-2–dependent. The VE-cadherin–dependent signals seem to be constitutively required, since basal levels of active Rac in VE-cadherin null cells were found to be lower than in WT cells.44 In the ICAM-2 null cells, on the other hand, Rac activation was normal at baseline but failed to increase during tube formation, suggesting that ICAM-2 provides a secondary signal required for junction stability via Rac activation. Preliminary results suggest that this may be the case: staining for JAM-A was reduced in monolayers from ICAM-2–/– migrating cells compared with WT cells (G.M.B., V.A., and A.M.R., unpublished observation). Detailed studies of the morphology of ICAM-2–/– cells during migration and junction formation and on the relationship between junctional pathways are in progress.
In summary, this study demonstrates that the leukocyte adhesion molecule ICAM-2 is involved in angiogenesis. We have identified a new pathway in which ICAM-2–mediated signaling activates the small GTPase Rac and regulates angiogenesis. We have described several new functions for ICAM-2, namely regulation of survival and cell migration and the ability to support homophilic interaction. This study also reveals a novel pathway for Rac activation in endothelial cells. Our results provide new insight in the regulation of endothelial homeostasis and angiogenesis by junctional adhesion molecules.
Prepublished online as Blood First Edition Paper, May 26, 2005; DOI 10.1182/blood-2004-12-4716.
Supported by the British Heart Foundation (A.M.R., D.O.H., M.-T.H., G.M.B.), Ludwig Institute for Cancer Research (A.J.R.), and European Union Network of Excellence MAIN (FP6-502935) (V.A.)
M.-T.H. performed research, generated reagents, data analysis and interpretation, contributed to design of research and writing the paper; J.C.M. performed research, contributed to data analysis and interpretation; G.M.B. performed research, contributed to data analysis and interpretation; V.A. performed research, contributed to data analysis and interpretation; N.G. provided key tools; D.O.H. contributed to design of research, data analysis and interpretation, and key tools; A.J.R. contributed to design of research, data analysis and interpretation, and key tools; and A.M.R. provided the original idea, designed research, contributed key tools and reagents, analyzed and interpreted data, and wrote the paper.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Britta Engelhardt (Theodor Kocher Institute, University of Bern, Switzerland) and Dr Sussan Nourshargh (Imperial College London, United Kingdom) for access to the ICAM-2–/– mice; Prof Yuti Chernajovsky (Barts and The London, United Kingdom) and Dr Charlotte Lawson (Imperial College London, United Kingdom) for the retrovirus-expressing cell line SVU19.5; Dr Alan Entwistle and Dr Priam Villalonga (Ludwig Institute, University College London, United Kingdom) and Dr Elaine Lidington (Imperial College London, United Kingdom) for helpful scientific and technical advice.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal