Abstract
The heparin-binding site of antithrombin is shown here to play a crucial role in mediating the antiangiogenic activity of conformationally altered cleaved and latent forms of the serpin. Blocking the heparin-binding site of cleaved or latent antithrombin by complexation with a high-affinity heparin pentasaccharide abolished the serpin's ability to inhibit proliferation, migration, capillary-like tube formation, basic fibroblast growth factor (bFGF) signaling, and perlecan gene expression in bFGF-stimulated human umbilical vein endothelial cells. Mutation of key heparin binding residues, when combined with modifications of Asn-linked carbohydrate chains near the heparin-binding site, also could abrogate the anti-proliferative activity of the cleaved serpin. Surprisingly, mutation of Lys114, which blocks anticoagulant activation of antithrombin by heparin, caused the native protein to acquire antiproliferative activity without the need for conformational change. Together, these results indicate that the heparin-binding site of antithrombin is of crucial importance for mediating the serpin's antiangiogenic activity and that heparin activation of native antithrombin constitutes an antiangiogenic switch that is responsible for turning off the antiangiogenic activity of the native serpin.
Introduction
Antithrombin is an abundantly expressed, key plasma protein regulator of blood coagulation in vertebrates.1 Inherited or acquired deficiencies of the protein in humans are associated with an increased risk of developing thrombotic disease,2 and complete deficiency in mice results in embryonic lethality due to a consumptive coagulopathy.3 Antithrombin regulates blood coagulation by directly inhibiting the serine proteases of the clotting cascade, the most important targets being thrombin, factor Xa, and factor IXa.1,4 The protein is a member of the serpin superfamily of protein protease inhibitors and shares a common tertiary structure with the family.5 It is unusual among serpins in requiring activation by the sulfated glycosaminoglycans, heparin or heparan sulfate, to inhibit its target proteases at a physiologically significant rate. A sequence-specific pentasaccharide present in only a fraction of heparin molecules mediates high-affinity binding and anticoagulant activation of antithrombin by the polysaccharide.1,6-8
In addition to its well-established anticoagulant function, anti-thrombin has more recently been shown to have anti-inflammatory,9 antiviral,10 and antiangiogenic functions.11,12 The antiangiogenic activity has been demonstrated from the ability to inhibit basic fibroblast growth factor (bFGF)– or vascular endothelial growth factor (VEGF)–induced endothelial cell proliferation, blood vessel growth in the chick embryo, and tumor growth in mice. The antitumor activity equals or exceeds that of other well-established antiangiogenic agents.13 Of importance, antiangiogenic activity is not present in native antithrombin but is expressed only after the protein undergoes conformational alterations induced by proteolytic cleavage in an exposed reactive loop or by mild heating.11,12 X-ray structures of these antiangiogenic forms of antithrombin have shown that the conformational alterations involve the insertion of the cleaved or intact reactive loop into the center of the major β-sheet of the protein core.14,15 The changes result in the loss of protease inhibitory activity and a large reduction in the affinity for heparin.16,17 Both conformationally altered antithrombin forms are produced under physiologic conditions, and therefore their antiangiogenic activity could have physiologic relevance.18,19
Because antiangiogenic antithrombins retain the ability to bind heparin, albeit weakly, we were interested in determining whether heparin binding was important for the expression of antiangiogenic activity. We provide evidence to show that a synthetic heparin pentasaccharide with high affinity for the antiangiogenic cleaved and latent forms of antithrombin antagonizes the antiangiogenic activity of the serpin as measured in proliferation, migration, capillary tube formation, growth factor signaling, and gene expression assays in bFGF-stimulated human umbilical vein endothelial cells (HUVECs). Mutation of heparin-binding site residues and the presence of Asn-linked carbohydrate chains adjacent to the heparin-binding site are shown to decrease the affinity of cleaved antithrombin for heparin and, when combined, to abolish the antiangiogenic activity. Most interesting, mutation of a pivotal heparin binding residue that critically determines the ability of native antithrombin to be conformationally activated by the anticoagulant pentasaccharide sequence is shown to result in the serpin acquiring antiangiogenic activity without the need for conformational change. These findings not only demonstrate the importance of the heparin-binding site in mediating antithrombin antiangiogenic activity, but also provide new insight into the conformational determinants of this activity.
Materials and methods
Antithrombin and heparins
Purified native and cleaved forms of plasma-derived human antithrombin were prepared as described previously.20 Recombinant wild-type and K125M, K114M, and R393W variant antithrombins were expressed in baby hamster kidney (BHK) cells and purified as described.21-23 The wild-type, K114M, and R393W variant antithrombins contained an additional N135Q mutation to eliminate glycosylation heterogeneity arising from incomplete glycosylation at Asn135.24 The corresponding glycoform of K125M antithrombin lacking carbohydrate at N135 was isolated.21 Both low- and high-heparin affinity glycoforms of wild-type and K114M recombinant antithrombins that were or were not fucosylated at the Asn 155 glycosylation site were isolated by heparin-agarose chromatography.25-27 The cleaved form of recombinant antithrombins was prepared by treatment with neutrophil elastase followed by successive heparin-Sepharose and MonoQ chromatography steps.20 Antithrombin concentrations were determined from the absorbance at 280 nm using an absorption coefficient of 37 700 M–1 cm–1 except for the R393W variant in which case a correction was made for the lost tryptophan residue.23,28 The purity of native and cleaved forms of all antithrombins was assessed by sodium dodecyl sulfate (SDS) and native polyacrylamide gel electrophoresis (PAGE).21,22
The natural heparin pentasaccharide that specifically binds antithrombin and a modified pentasaccharide with much higher affinity for the serpin29 (respectively compounds 39 and 92 in Van Boeckel and Petitou30 ) were generously provided by Dr Maurice Petitou of Sanofi Recherche (Toulouse, France). A full-length heparin of approximately 50 saccharides and containing the pentasaccharide was isolated from commercial heparin by size and antithrombin-affinity fractionation as described.28
Heparin binding assays
Binding of heparin pentasaccharides to native, cleaved, and latent forms of antithrombin was quantified by titrating solutions of antithrombin (0.5 μM), containing the fluorescence probe 2-(p-toluidinyl)naphthalene-6-sulfonic acid (TNS; 10 μM), with heparin and monitoring the 50% to 60% quenching of bound TNS fluorescence that accompanies heparin binding.31 Titrations were performed at 25°C in a physiologic ionic strength (I 0.15) and pH buffer (pH 7.4) consisting of 20 mM sodium phosphate, 0.1 M NaCl, 0.1 mM EDTA (ethylenediaminetetraacetic acid), and 0.1% polyethylene glycol (PEG) 8000. Titrations were fit by nonlinear regression to the quadratic equilibrium binding equation to obtain values for the binding stoichiometry, dissociation constant (KD) and ΔFmax/Fo,28 except for the binding of the natural pentasaccharide to cleaved and latent antithrombins, in which case a 1:1 stoichiometric interaction was assumed.
The relative heparin affinities of plasma and recombinant antithrombins in native and cleaved forms were evaluated from the salt concentration required to elute the protein from a 5-mL Hi-Trap heparin column (Amersham Biosciences). Proteins (∼ 10 μg) were applied to the column after equilibration with 20 mM sodium phosphate, 0.1 mM EDTA buffer, pH 7.4. The column was washed with this buffer for 15 minutes at a flow rate of 1 mL/min, and then a linear salt gradient to 3 M NaCl was applied over the next 45 minutes. For proteins eluting at NaCl concentrations under 0.5 M, a 2-stage gradient was applied, first to 0.5 M NaCl over 25 minutes and then to 3 M NaCl over the next 20 minutes. Elution of the protein was continuously monitored by protein fluorescence.
Cell culture
HUVECs (American Type Culture Collection [ATCC], Manassas, VA) were cultured in F-12K medium supplemented with 10% fetal calf serum (Invitrogen, Frederick, MD), 1% antibiotics (penicillin and streptomycin), 15 μg/mL endothelial growth supplement (Sigma, St Louis, MO), and 10 μg/mL heparin (Sigma) at 37°C, 5% CO2 in air. HUVECs were used within 10 passages.
Cell-proliferation assay
HUVECs were seeded in 96-well plates at a density of 5000 cells per well in reduced fetal bovine serum (FBS, 0.5%) starving F-12K media. After 16 hours, cells were exposed to 10 ng/mL bFGF (R&D Systems, Minneapolis, MN) in the presence or absence of antithrombin (20 μg/mL) for 48 hours. Cells were then incubated with 20 μL methyl-thiazol tetrazolium (MTT) solution (Promega, Madison, WI) for 1 to 3 hours at 37°C and the absorbance at 490 nm was measured.
Wound-induced migration assays
Migration assays were performed as described.32 Cells suspended in F-12K medium with 10% FBS were seeded onto 12-well plastic plates coated with fibronectin (2 × 105 cells/well) and incubated for 24 hours. Subconfluent monolayers of the cells were then scraped with a plastic pipette tip, washed with warm phosphate-buffered saline (PBS) twice, and the medium was replaced with serum-free F-12K with or without bFGF and antithrombin. Cells were photographed at 0 and 6 hours after scraping.
Chemotaxis assays
Chemotaxis assays were performed in a 48-well modified Boyden chamber (Neuroprobe, Gaithersburg, MD) as described.33 Nitrocellulose filters (8-μm–pore sized; Corning, Cambridge, MA) were coated with 100 μg/mL gelatin (Collaborative Biomedical Products, Bedford, MA). HUVECs were preincubated in F-12K medium containing 0.5% FBS with or without antithrombin (20-30 μg/mL) for 40 minutes, washed twice, and then suspended at 7 × 105 cells/mL in the same medium. HUVECs (30-38 μL) were added to the lower chamber of each well and 58 μL F-12K medium containing bFGF and 0.1% bovine serum albumin was added to the upper chambers. The assembled chemotaxis chamber was turned upside down and incubated for 2 hours at 37°C with 5% CO2. Nonmigrated cells on the upper side of the filter were removed by scraping, and the filter was stained with Diff-Quick (VWR Scientific Products, Bridgeport, NJ). Migrated cells on the lower side of the filter were counted in 3 separate fields and photographed. Assays were run in quadruplicate.
Capillary-like tube formation on matrigel
Differentiation of HUVECs to capillary tubes was examined as published.34 Growth factor–reduced matrigel (250 μL; BD Science, San Diego, CA) at 4°C was transferred to a cold 24-well plate (Falcon; Becton Dickinson, Oxford, United Kingdom) and allowed to polymerize at 37°C for 30 minutes. Washed HUVECs were suspended in endothelial cell basal medium-2 (EBM; Clonetics, Palo Alto, CA) containing 50 ng/mL bFGF, and 40 000 cells were transferred to each well of the matrigel-coated plate. After 1 to 2 hours of incubation at 37°C with 5% CO2 and 95% humidity to allow cell attachment, 125 μL antithrombin (20-50 μg/mL) dissolved in EBM was added and the plates were incubated for an additional 4 to 16 hours. The medium was then decanted and cells were fixed and stained using a modified Hema 3 Stain kit (Fisher, Swedesboro, NJ). Capillary-like tubes were observed and photographed using light microscopy.
Protein extraction, Western blotting, and immunoprecipitation
Cells were suspended and sonicated in nonidet P-40 lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.5, 0.5% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 0.5 mM sodium orthovanadate; Sigma), 10% glycerol, and complete protease-inhibitor cocktail [Boehringer Mannheim, Indianapolis, IN]). Extracts were centrifuged at 10 000g for 20 minutes, and the total protein content in supernatants was determined by the bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL). Equal amounts of total protein (50-100 μg) were resolved by SDS-PAGE and then transferred to Immobilon-P transfer membranes (Millipore, Marlborough, MA). Nonspecific binding sites were blocked by incubation for 1 hour with Tris-buffered saline (TBS) containing 0.075% Tween-20 (TBST) and 5% nonfat dry milk. Membranes were exposed to primary antibodies in TBST–0.5% milk for 1 to 2 hours followed by incubation with horseradish peroxidase–conjugated secondary antibodies. Immunoreactivity was visualized by Supersignal West Pico chemiluminescence substrate (Pierce Biotechnology). For assessment of differences in protein loading, the membranes were incubated at 50°C for 45 minutes with 2-mercaptoethanol (100 mM) and SDS (2%) in Tris-HCl (62 mM, pH 6.7), blocked, and incubated with an anti–pan–mitogen-activated protein kinase (MAPK) immunoglobulin G (IgG; Cell Signaling, Beverly, MA) or an anti–β-actin IgG (Sigma). For FGF receptor-1 (FGFR-1) immunoprecipitation studies, 200 μg total protein extract was precleared with immobilized protein A Sepharose beads (Pierce) and then incubated with 4 μg/mL antiphosphotyrosine mouse monoclonal antibody (Upstate, Lake Placid, NY) in 200 μL lysis buffer for 4 hours on ice. The immune complexes were precipitated for 4 hours with 50 μL of a 50% slurry of protein A Sepharose at 4°C. Immunoprecipitated complexes were washed 3 times with lysis buffer containing 0.5% nonidet P-40, resuspended in SDS sample buffer, boiled for 5 minutes, and resolved by SDS-PAGE. Subsequent immunoblots were probed with polyclonal antibodies against FGFR-1 (Upstate).
Electrophoretic mobility shift assay
The oligonucleotide corresponding to the transforming growth factor β (TGFβ) binding site of the perlecan promoter (5′-TGGCCCGGCGGCCC)35 was synthesized (Sigma) and 32P-labeled by incubating with [32P]–adenosine triphosphate (ATP; Amersham) and T4 kinase (Promega) at 37°C for 30 minutes. The labeled oligonucleotide probe was separated from free [32P]-ATP by Sephadex G50 chromatography. Nuclear extract protein (10-15 μg) prepared from treated HUVECs, essentially by the method of Dignam et al,36 plus 1 μg poly (dI-dC) (Pharmacia, Piscataway, NJ) were incubated with various concentrations of unlabeled oligonucleotide probe in 20 mM Tris buffer, pH 7.8, containing 1 mM MgCl2, 12% glycerol, and 0.1 mM dithiothreitol (DTT) for 25 minutes at 4°C. 32P-labeled oligonucleotide probe (0.5-1.0 ng equal to 20 000-40 000 cpm) was then added to each reaction and further incubated for 30 minutes at 25°C. The sample was electrophoresed in 5% nondenaturing polyacrylamide gels in Tris-borate-EDTA at a constant 180 V for 4 hours at 4°C. The gel was subsequently dried, and bands were visualized by autoradiography.
Results
Characterization of a pentasaccharide probe of the heparin-binding site of antiangiogenic antithrombin
A synthetic heparin pentasaccharide designed to bind native antithrombin with higher affinity than the natural pentasaccharide sequence29,30 was tested for its ability to bind antiangiogenic forms of antithrombin. Equilibrium binding titrations of native and cleaved antithrombins with the high-affinity pentasaccharide variant monitored by TNS fluorescence changes31 showed that the saccharide bound both antithrombins with 1:1 stoichiometries and KDs of approximately 1 and 50 nM, respectively (Figure 1A). Latent antithrombin bound the saccharide with an affinity similar to the cleaved form. These KDs reflected considerably higher binding affinities than the KDs of approximately 50 nM and 1000 to 2000 nM measured for the natural pentasaccharide binding to native antithrombin and to cleaved or latent antithrombins, respectively. While the KD for the native antithrombin-pentasaccharide interaction agrees with past reports,7,24 weaker affinities of cleaved and latent antithrombins for the natural pentasaccharide were measured in a past report using intrinsic protein fluorescence changes to monitor binding.37 Native PAGE confirmed that the high-affinity pentasaccharide formed complexes with both cleaved and native forms of antithrombin as shown by the electrophoretic mobility shift of the protein induced by the addition of saccharide (Figure 1B). The natural pentasaccharide or a full-length heparin containing the pentasaccharide produced little or no shift in either native or cleaved antithrombin bands in the electrophoretic assay. These results showed that the high-affinity pentasaccharide could be used to assess the importance of the heparin-binding site of cleaved antithrombin in mediating the serpin's antiangiogenic activity.
High-affinity pentasaccharide effects on antithrombin antiangiogenic activity
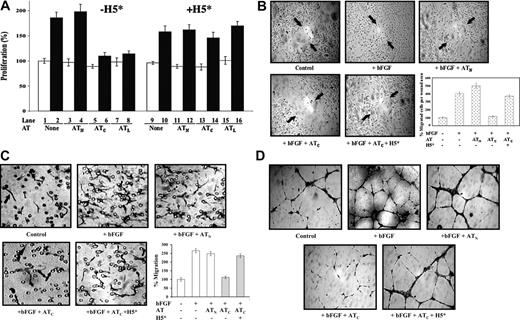
We first tested whether the high-affinity pentasaccharide affected the ability of antiangiogenic cleaved antithrombin to inhibit the proliferation of cultured HUVECs. Cleaved antithrombin but not the native protein inhibited bFGF-stimulated HUVEC proliferation as in previous studies (Figure 2A).11,20 This inhibition was completely abrogated when a near-stoichiometric amount of high-affinity pentasaccharide was added (Figure 2A), but not when the natural pentasaccharide or the full-length heparin was included at the same levels (data not shown). All 3 forms of heparin did not appear to have any other effects on HUVEC proliferation at the levels used. The antiangiogenic latent form of antithrombin similarly inhibited bFGF-stimulated proliferation of HUVECs, and this inhibition was also reversed by the high-affinity pentasaccharide (Figure 2A). A reactive loop mutant of antithrombin in which the P1 arginine, a residue critical for the anticoagulant function of the serpin, was changed to tryptophan23 showed the same bFGF-dependent antiproliferative activity toward HUVECs as the wild-type protein when the reactive loop was cleaved but not with the uncleaved native protein. This activity was again reversed by the high-affinity pentasaccharide (not shown). The antiproliferative activity of cleaved and latent forms of antithrombin thus requires the heparin-binding site but is independent of the reactive loop P1 residue that is essential for the anticoagulant function of the native serpin. Native antithrombin failed to show any inhibition of HUVEC growth at levels as high as 500 μg/mL. Such levels far exceed the reported number of anticoagulant pentasaccharide binding sites in endothelial cell heparan sulfate,38 suggesting that binding of native antithrombin to nonanticoagulant heparan sulfate sites in HUVECs39 was not sufficient to mediate antiangiogenic activity.
High-affinity pentasaccharide forms a complex with cleaved antithrombin. (A) Fluorescence titrations of 0.5 μM native (▴ and ▵) or cleaved (• and ○) antithrombins with natural pentasaccharide (H5) (▴ and •) or the high-affinity pentasaccharide (H5*) (▵ and ○) in the presence of 10 μM TNS monitored by TNS fluorescence changes. Fluorescence changes are expressed relative to the maximal fluorescence change at saturation obtained from nonlinear regression fits to the equilibrium binding equation (solid lines). (B) Samples of 6 μM native or cleaved antithrombin in the absence or presence of 12 μM full-length heparin (H50), natural pentasaccharide (H5), or high-affinity pentasaccharide (H5*) were subjected to 10% native PAGE and stained with Coomassie blue dye.
High-affinity pentasaccharide forms a complex with cleaved antithrombin. (A) Fluorescence titrations of 0.5 μM native (▴ and ▵) or cleaved (• and ○) antithrombins with natural pentasaccharide (H5) (▴ and •) or the high-affinity pentasaccharide (H5*) (▵ and ○) in the presence of 10 μM TNS monitored by TNS fluorescence changes. Fluorescence changes are expressed relative to the maximal fluorescence change at saturation obtained from nonlinear regression fits to the equilibrium binding equation (solid lines). (B) Samples of 6 μM native or cleaved antithrombin in the absence or presence of 12 μM full-length heparin (H50), natural pentasaccharide (H5), or high-affinity pentasaccharide (H5*) were subjected to 10% native PAGE and stained with Coomassie blue dye.
Since angiogenesis is a complex physiologic process involving endothelial cell proliferation, migration, and differentiation to form capillary tubes with associated changes in extracellular matrix,40 it was important to determine whether the high-affinity heparin pentasaccharide had similar abrogating effects on antithrombin antiangiogenic activity in other biologic assays of this activity. The effects of native and cleaved antithrombins on the bFGF-stimulated migration of cultured HUVECs after inducing a scratch wound32 were examined (Figure 2B). bFGF dramatically enhanced the migration of HUVECs in this assay and cleaved antithrombin significantly suppressed this enhanced migration, whereas native antithrombin had no effect. Again, the high-affinity pentasaccharide blocked the suppression of HUVEC migration by cleaved antithrombin, whereas natural pentasaccharide and full-length heparins had no effect.
High-affinity pentasaccharide abrogates the inhibitory effects of cleaved antithrombin on bFGF-induced HUVEC proliferation, wound healing, migration, and capillary tube formation. Resting HUVECs were cultured in reduced serum F-12K media and treated with bFGF (10 ng/mL) and native (N), cleaved (C), or latent (L) forms of antithrombin (AT, 20-50 μg/mL) in the presence or absence of 0.25 μM high-affinity pentasaccharide (H5*) as indicated. (A) Measurements of HUVEC proliferation after 48-hour incubation by the addition of MTT reagent and measurement of absorbance at 490 nm as in previous studies.20 Cells were unstimulated (□) or stimulated with bFGF (▪) in the absence or presence of the indicated antithrombin forms. Experiments were performed both in the absence (lanes 1-8) and presence (lanes 9-16) of the high-affinity pentasaccharide. Each value represents the mean of 3 experiments ± SD in each of which quadruplicate samples were run. (B) Wound-induced migration assay using confluent resting HUVECs wounded by scratching and further cultured with bFGF and antithrombin in the presence or absence of H5* for 4 hours. Arrows indicate the original wound edge. Magnification, × 100. The mean ± SD number of cells that had migrated into the wound area was quantitated by counting in 3 random areas in 2 independent experiments. (C) Chemotaxis assay for the migration of HUVECs cultured with antithrombin with or without H5* toward bFGF. Migrated cells appear as elongated spindle shapes (black arrow). The circular structures represent pores in the membrane (black arrow). Magnification, × 200. The mean ± SD number of migrated cells for each condition was quantitated by counting 3 independent fields at × 100 magnification. Samples were run in quadruplicate and the results reflect 2 separate experiments. (D) Subconfluent HUVECs plated on matrigel were treated as indicated. After a 16-hour incubation at 37°C, cells were stained and photographed. Magnification × 200. Images in panels B-D were obtained with a Leica Microsystems DMLB microscope and camera (Leica, Wetzlar, Germany). A water-immersion 20 ×/0.3-0.5 NA objective lens was used.
High-affinity pentasaccharide abrogates the inhibitory effects of cleaved antithrombin on bFGF-induced HUVEC proliferation, wound healing, migration, and capillary tube formation. Resting HUVECs were cultured in reduced serum F-12K media and treated with bFGF (10 ng/mL) and native (N), cleaved (C), or latent (L) forms of antithrombin (AT, 20-50 μg/mL) in the presence or absence of 0.25 μM high-affinity pentasaccharide (H5*) as indicated. (A) Measurements of HUVEC proliferation after 48-hour incubation by the addition of MTT reagent and measurement of absorbance at 490 nm as in previous studies.20 Cells were unstimulated (□) or stimulated with bFGF (▪) in the absence or presence of the indicated antithrombin forms. Experiments were performed both in the absence (lanes 1-8) and presence (lanes 9-16) of the high-affinity pentasaccharide. Each value represents the mean of 3 experiments ± SD in each of which quadruplicate samples were run. (B) Wound-induced migration assay using confluent resting HUVECs wounded by scratching and further cultured with bFGF and antithrombin in the presence or absence of H5* for 4 hours. Arrows indicate the original wound edge. Magnification, × 100. The mean ± SD number of cells that had migrated into the wound area was quantitated by counting in 3 random areas in 2 independent experiments. (C) Chemotaxis assay for the migration of HUVECs cultured with antithrombin with or without H5* toward bFGF. Migrated cells appear as elongated spindle shapes (black arrow). The circular structures represent pores in the membrane (black arrow). Magnification, × 200. The mean ± SD number of migrated cells for each condition was quantitated by counting 3 independent fields at × 100 magnification. Samples were run in quadruplicate and the results reflect 2 separate experiments. (D) Subconfluent HUVECs plated on matrigel were treated as indicated. After a 16-hour incubation at 37°C, cells were stained and photographed. Magnification × 200. Images in panels B-D were obtained with a Leica Microsystems DMLB microscope and camera (Leica, Wetzlar, Germany). A water-immersion 20 ×/0.3-0.5 NA objective lens was used.
To further assess the effects of the high-affinity pentasaccharide on the inhibition of bFGF-induced cell migration by cleaved antithrombin, we performed a chemotaxis-type migration assay using a mini-Boyden chamber.33 bFGF significantly stimulated HUVECs to migrate (up to 270%) compared with the non–bFGF-treated control (Figure 2C). Native antithrombin had no significant effect on the bFGF-induced cell migration, but cleaved antithrombin inhibited the bFGF-stimulated migration by 70% to 80%. As with the other antiangiogenic assays, the high-affinity heparin pentasaccharide significantly reversed (up to 90%) the inhibition of bFGF-induced cell migration by cleaved antithrombin.
Finally, the ability of heparin to block antithrombin's antiangiogenic activity was examined in a capillary tube formation assay (Figure 2D).34 bFGF induced HUVECs to differentiate and form capillary tubes on matrigel. Cleaved antithrombin significantly reduced the number and dimensions of capillary tubes induced by bFGF, whereas native antithrombin did not significantly affect tube formation. Again, the high-affinity heparin pentasaccharide was found to abrogate the inhibition of bFGF-induced capillary tube formation by cleaved antithrombin.
Effects of antiangiogenic antithrombin and high-affinity pentasaccharide on bFGF-dependent signaling in HUVECs
To determine the effects of antiangiogenic antithrombin on bFGF-induced signaling in endothelial cells, we examined growth factor–induced autophosphorylation of FGF receptor-1 (FGFR-1) and phosphorylation of MAP kinase in HUVECs.41-43 The majority of MAP kinase was in an inactive dephosphorylated state in resting HUVECs but was significantly activated by tyrosine phosphorylation after treatment with bFGF (Figure 3A). bFGF stimulation of MAP kinase phosphorylation was inhibited in HUVECs treated with cleaved antithrombin but not with native antithrombin. Neither antithrombin nor bFGF affected MAP kinase gene expression as shown by the similar levels of total MAP kinase protein in treated and untreated HUVECs. That the heparin-binding site of antithrombin was critical for its inhibition of FGF receptor signaling was shown by the ability of the high-affinity heparin pentasaccharide to attenuate the inhibition of MAP kinase phosphorylation by cleaved antithrombin.
Similar inhibitory effects of cleaved antithrombin and reversal of these effects by the high-affinity pentasaccharide were observed on bFGF-induced autophosphorylation of FGFR-1 (Figure 3B). The majority of FGFR-1 was in an inactive unphosphorylated form in resting HUVECs. bFGF dramatically elevated the level of autophosphorylated FGFR-1 in HUVECs. This bFGF-enhanced autophosphorylation of FGFR-1 was suppressed in cells treated with cleaved antithrombin but not with native antithrombin. The high-affinity pentasaccharide by itself did not affect bFGF-induced phosphorylation of FGFR-1, but it clearly attenuated the inhibition of bFGF-dependent FGFR-1 autophosphorylation by cleaved antithrombin. Together, these results indicated that the heparin-binding site of antithrombin was critical for the cleaved serpin to inhibit bFGF-initiated signaling through phosphorylation of FGFR-1 and MAP kinase.
High-affinity pentasaccharide attenuates the inhibitory effects of cleaved antithrombin on bFGF-induced MAP kinase and FGFR-1 phosphorylation in HUVECs. Resting HUVECs (5 × 105) were incubated with 20 μg/mL native or cleaved forms of antithrombin in the presence and absence of 0.25 μM H5* in reduced FBS medium for 2 hours at 37°C. Then, 20 ng/mL bFGF was applied to stimulate the cells for 10 minutes at 37°C. (A) Cell extracts were prepared and resolved by 10% SDS-PAGE and then immunoblotted with either an anti–phosphospecific MAPK IgG (p42/44-MAPK) or an anti–pan-MAPK IgG (42/44-MAPK). The ratio of phosphorylated to total MAPK was quantified from the band intensities with a Kodak Image Station 440-CF (Kodak, Rochester, NY). (B) Equal amounts (200 μg) of cell extract protein were immunoprecipitated with a phospho-tyrosine–specific antibody and were subsequently immunoblotted with an antibody specific for FGFR-1. The results shown are from 1 of 3 experiments that all gave similar results.
High-affinity pentasaccharide attenuates the inhibitory effects of cleaved antithrombin on bFGF-induced MAP kinase and FGFR-1 phosphorylation in HUVECs. Resting HUVECs (5 × 105) were incubated with 20 μg/mL native or cleaved forms of antithrombin in the presence and absence of 0.25 μM H5* in reduced FBS medium for 2 hours at 37°C. Then, 20 ng/mL bFGF was applied to stimulate the cells for 10 minutes at 37°C. (A) Cell extracts were prepared and resolved by 10% SDS-PAGE and then immunoblotted with either an anti–phosphospecific MAPK IgG (p42/44-MAPK) or an anti–pan-MAPK IgG (42/44-MAPK). The ratio of phosphorylated to total MAPK was quantified from the band intensities with a Kodak Image Station 440-CF (Kodak, Rochester, NY). (B) Equal amounts (200 μg) of cell extract protein were immunoprecipitated with a phospho-tyrosine–specific antibody and were subsequently immunoblotted with an antibody specific for FGFR-1. The results shown are from 1 of 3 experiments that all gave similar results.
Effects of antiangiogenic antithrombin and high-affinity pentasaccharide on perlecan gene expression
We previously demonstrated that antiangiogenic forms of antithrombin inhibit the proliferation of HUVECs at least partially by suppressing the expression of the matrix and cell surface heparan sulfate proteoglycan, perlecan,20 a well-established proangiogenic factor.44,45 Nuclear factor-1 (NF-1) binding to the TGF-β cis-element in the perlecan promoter correlates with perlecan gene expression levels35 and was used to assess the expression level of the perlecan gene in HUVECs by electrophoretic mobility shift assay. A substantial amount of NF-1 was bound by the TGF-β–responsive probe in nuclear extracts of bFGF-stimulated HUVECs, and native antithrombin did not significantly affect this binding (Figure 4). However, cleaved antithrombin completely blocked the formation of NF-1–probe complex in the nuclear extracts of bFGF-treated HUVECs. Inclusion of the high-affinity pentasaccharide but not the natural pentasaccharide in HUVECs treated with bFGF and cleaved antithrombin caused the amount of NF-1–TGF-β probe complex to return to the control level. The high-affinity pentasaccharide alone did not affect the amount of complex formed. That NF-1–probe complexes were being observed was shown by the supershift of the complex band when an anti–NF-1 antibody was added to the nuclear extract–probe mixture. When TGF-β but not TGF-α was added to HUVECs treated with bFGF, the amount of NF-1–TGF-β probe complex was elevated to levels even greater than the control, confirming that the probe sequence used in the assay was specifically responsive to TGF-β stimulation of the cells.35 Addition of TGF-β to bFGF-stimulated cells treated with cleaved antithrombin overcame the inhibitory effect of the cleaved serpin on NF-1–probe complex formation (not shown). Together, these results confirmed our previous findings that cleaved antithrombin produces its antiproliferative effect on bFGF-stimulated HUVECs at least in part by down-regulating the expression of perlecan,20 and further suggested that the heparin-binding site of antithrombin is required for this down-regulation.
Correlation of antithrombin antiangiogenic activity with heparin binding affinity
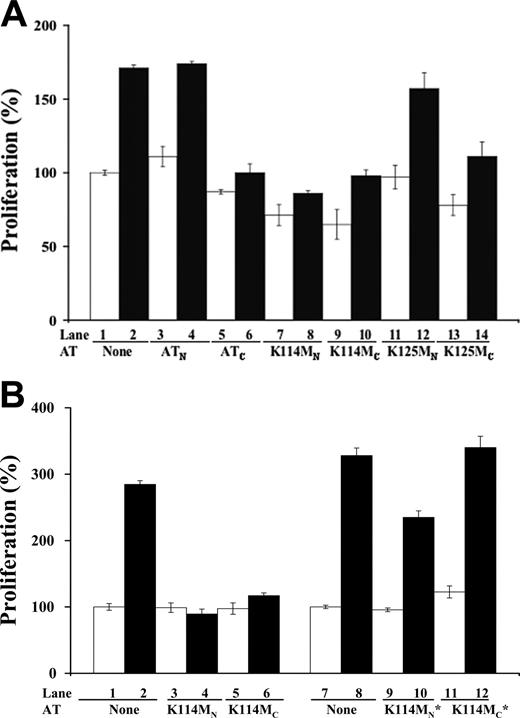
To further test the importance of the heparin-binding site of antithrombin in mediating the serpin's antiangiogenic activity, we determined whether mutations in key heparin binding residues affected antiangiogenic activity. The mutations were done on a wild-type background corresponding to the beta form of plasma antithrombin that is not glycosylated at the Asn135 site in order to eliminate glycosylation heterogeneity at this site and its consequent effects on heparin affinity.24-27 Mutations of Lys125 or Lys114 to Met result in substantial 200-fold and 105-fold reductions in the affinity of native antithrombin for heparin, respectively.21,22 These mutations reduced the affinity of cleaved antithrombin for heparin as well, as judged from the decreased salt concentrations at which the cleaved mutants eluted from heparin-agarose relative to the cleaved wild-type serpin (Table 1). However, the Lys125 and Lys114 mutations caused comparable losses in the affinity of cleaved antithrombin for heparin, in sharp contrast to the very different heparin affinity losses produced by these mutations in native antithrombin (Table 1). The cleaved form of K125M antithrombin showed at most a modest change in antiproliferative activity in bFGF-stimulated HUVECs compared with cleaved wild-type antithrombin, whereas the native form of the mutant did not significantly affect HUVEC proliferation (Figure 5A). The cleaved form of K114M antithrombin also showed little change in the ability to inhibit bFGF-stimulated HUVEC proliferation. Unexpectedly, however, the native form of this mutant was found to acquire the ability to inhibit bFGF-induced proliferation like the cleaved form (Figure 5A).
Relative heparin affinities of native and cleaved forms of plasma and recombinant antithrombins
. | NaCl concentration at peak protein elution, M . | . | |
|---|---|---|---|
| Antithrombin . | Native . | Cleaved . | |
| Plasma wild type | 1.43 ± 0.01 | 0.43*† | |
| r-wild-type HA glycoform | 2.09* | 0.67 ± 0.02 | |
| r-K125M HA glycoform | 1.62‡ | 0.29 ± 0.02 | |
| r-K114M HA glycoform | 0.29 ± 0.01 | 0.36 ± 0.01 | |
| r-wild-type LA glycoform | 1.38 ± 0.02 | 0.71 ± 0.05 | |
| r-K114M LA glycoform | 0.26 ± 0.01 | 0.33 ± 0.02 | |
. | NaCl concentration at peak protein elution, M . | . | |
|---|---|---|---|
| Antithrombin . | Native . | Cleaved . | |
| Plasma wild type | 1.43 ± 0.01 | 0.43*† | |
| r-wild-type HA glycoform | 2.09* | 0.67 ± 0.02 | |
| r-K125M HA glycoform | 1.62‡ | 0.29 ± 0.02 | |
| r-K114M HA glycoform | 0.29 ± 0.01 | 0.36 ± 0.01 | |
| r-wild-type LA glycoform | 1.38 ± 0.02 | 0.71 ± 0.05 | |
| r-K114M LA glycoform | 0.26 ± 0.01 | 0.33 ± 0.02 | |
Plasma and recombinant (r) antithrombins, including both low-heparin affinity (LA) and high-heparin affinity (HA) glycoforms of the latter, were chromatographed on heparin-agarose as detailed in “Materials and methods.” All antithrombin forms were chromatographed at least twice except where noted, and average values for the salt concentrations at which the protein peak was eluted are reported with errors representing the range of observed values.
Identical values were obtained in 2 chromatography runs.
Latent antithrombin was eluted from the column at a similar value of 0.40 ± 0.01 M NaCl.
Value from a single chromatography run due to limited amounts of the sample.
High-affinity pentasaccharide abolishes the effects of cleaved anti-thrombin on perlecan gene expression. Electrophoretic mobility shift assay of a 32P-labeled DNA oligonucleotide probe of the TGF-β binding element of the perlecan gene promoter after incubation with nuclear extracts obtained from resting HUVECs pretreated with native or cleaved forms of antithrombin (20 μg/mL) and bFGF (10 ng/mL) in the presence or absence of 0.25 μM natural pentasaccharide (H5) or the high-affinity pentasaccharide (H5*) for 48 hours. TGF-β1 or TGF-α (both 1 ng/mL; Sigma) was added to the cells at 4 hours before harvesting as positive and negative controls for the induction of perlecan gene expression. The identity of the NF-1–probe complex band was confirmed from the supershifting of the complex band when anti–NF-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was included in the incubation.
High-affinity pentasaccharide abolishes the effects of cleaved anti-thrombin on perlecan gene expression. Electrophoretic mobility shift assay of a 32P-labeled DNA oligonucleotide probe of the TGF-β binding element of the perlecan gene promoter after incubation with nuclear extracts obtained from resting HUVECs pretreated with native or cleaved forms of antithrombin (20 μg/mL) and bFGF (10 ng/mL) in the presence or absence of 0.25 μM natural pentasaccharide (H5) or the high-affinity pentasaccharide (H5*) for 48 hours. TGF-β1 or TGF-α (both 1 ng/mL; Sigma) was added to the cells at 4 hours before harvesting as positive and negative controls for the induction of perlecan gene expression. The identity of the NF-1–probe complex band was confirmed from the supershifting of the complex band when anti–NF-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was included in the incubation.
Because of the potentially confounding effect of eliminating the carbohydrate chain at the Asn135 site on the heparin affinity and antiangiogenic activity of the mutant recombinant antithrombins (Table 1), we also examined a lower heparin affinity glycoform produced by the mammalian expression system as a result of fucosylation of the nearby Asn155-linked carbohydrate chain.25-27 Fucosylation, surprisingly, was found to have small effects on the heparin affinity of cleaved wild-type antithrombin as well as both native and cleaved forms of K114M antithrombin, in contrast to its large effect on the affinity of native wild-type antithrombin (Table 1). Fucosylation thus appeared to specifically affect the high-heparin affinity interaction produced by conformational activation of native antithrombin. Both fucosylated and nonfucosylated recombinant wild-type antithrombin glycoforms behaved like plasma antithrombin in showing inhibition of bFGF-dependent HUVEC proliferation by the cleaved form and no effect of the native form of the protein (not shown). By contrast, the low-heparin affinity fucosylated form of cleaved K114M antithrombin showed a complete loss in the ability to inhibit bFGF-dependent HUVEC proliferation (Figure 5B). The low-heparin affinity glycoform of native K114M antithrombin also appeared to lose much of the antiproliferative activity that was observed in the high-heparin affinity glycoform. The corresponding low-heparin affinity forms of the K125M mutant were not examined because they were a mixture of forms either glycosylated at Asn135 or fucosylated at Asn155. That the observed growth inhibitory effects of all wild-type and mutant antithrombins on HUVECs required the heparin-binding site was shown by the reversal of growth inhibition by the high-affinity pentasaccharide (not shown). Together, these results suggested that mutation of either Lys125 or Lys114 alone is insufficient to abolish the antiangiogenic activity of cleaved antithrombin in the context of a beta-type plasma antithrombin lacking the Asn135 carbohydrate chain. However, mutation of Lys114 can abolish antiangiogenic activity in this context when the nearby Asn155 carbohydrate chain is fucosylated. More intriguing, the findings implied that blocking heparin activation of native antithrombin by mutation of Lys114 impairs a conformational switch that is responsible for turning off the antiangiogenic activity of native antithrombin.
Discussion
A number of antiangiogenic proteins are known to bind heparin, and this binding appears to be important for their antiangiogenic function.46,47 Our findings support the conclusion that heparin binding to the antiangiogenic cleaved or latent forms of antithrombin is crucial for the serpin to express its antiangiogenic activity. This is indicated from our observations that the antiangiogenic activity of cleaved and latent antithrombins is abolished when the heparin-binding site is blocked with a high-affinity heparin pentasaccharide or when modifications are made in heparin-binding site residues and Asn-linked carbohydrate chains that affect heparin affinity. Multiple biologic assays of antithrombin antiangiogenic activity including endothelial cell proliferation, migration, capillary tube formation, bFGF-dependent signaling, and perlecan gene expression were used to provide a robust demonstration of the importance of the heparin-binding site of antithrombin in mediating the many manifestations of the serpin's antiangiogenic activity.11,12,20
Our major conclusion hinges on the assumption that the high-affinity pentasaccharide specifically blocks the heparin-binding site of cleaved antithrombin. The high-affinity pentasaccharide is known to specifically bind to the same electropositive site on native antithrombin as the natural heparin pentasaccharide.29,36 This pentasaccharide was also shown to form a high-affinity stoichiometric complex with the cleaved antiangiogenic form of antithrombin by electrophoretic mobility shift assay and fluorescence equilibrium binding assays in the present study. The x-ray structure of a similar high-affinity pentasaccharide complexed with a dimer of latent and native antithrombin has shown that the high-affinity pentasaccharide specifically interacts at the same site in both native and antiangiogenic latent forms of antithrombin despite the different conformations of these forms and their different affinities for the saccharide.8 This binding specificity together with our finding that stoichiometric quantities of the saccharide suffice to ablate the antiangiogenic activity of cleaved and latent antithrombins on growth factor–stimulated HUVECs without affecting endothelial cells in the absence of antithrombin strongly suggest that the antagonistic effects of the high-affinity pentasaccharide result from the saccharide binding to and blocking the heparin-binding site of the cleaved or latent serpin. The natural pentasaccharide or a full-length heparin containing this sequence was unable to block the antiangiogenic activity of cleaved antithrombin presumably because of their much lower affinity for the cleaved protein. Such heparins were shown in this and a past study to bind cleaved antithrombin with KDs in the range 10–6 to 10–5 M, which are several orders of magnitude weaker than the affinity for native antithrombin16,37 and would therefore be insignificantly bound at the levels used in our assays. It follows that a low-affinity interaction of heparin with antiangiogenic cleaved or latent forms of antithrombin is required for the protein to express its antiangiogenic activity. Presumably this requirement is met by a cell surface or matrix heparan sulfate proteoglycan present in cultured endothelial cells.
Effects of heparin-binding site mutations on antithrombin antiangiogenic activity. Resting HUVECs were incubated with 20 μg/mL native (N) and cleaved forms (C) of wild-type, K114M, and K125M antithrombins in the presence (▪) and absence (□) of bFGF (10 ng/mL) for 48 hours. Panel A shows the effect of high-affinity glycoforms of the recombinant antithrombins, whereas panel B compares high-affinity with low-affinity glycoforms of the K114M variant (the latter denoted by *). Colorimetric quantification of cell numbers was achieved as described in Figure 2A. Each value represents the mean of 3 experiments ± SD in each of which quadruplicate samples were run.
Effects of heparin-binding site mutations on antithrombin antiangiogenic activity. Resting HUVECs were incubated with 20 μg/mL native (N) and cleaved forms (C) of wild-type, K114M, and K125M antithrombins in the presence (▪) and absence (□) of bFGF (10 ng/mL) for 48 hours. Panel A shows the effect of high-affinity glycoforms of the recombinant antithrombins, whereas panel B compares high-affinity with low-affinity glycoforms of the K114M variant (the latter denoted by *). Colorimetric quantification of cell numbers was achieved as described in Figure 2A. Each value represents the mean of 3 experiments ± SD in each of which quadruplicate samples were run.
Support for the involvement of the heparin-binding site of antithrombin in expressing the antiangiogenic activity of the cleaved serpin was provided by studies of variant antithrombins with mutations in the heparin-binding site or modifications of nearby glycosylation sites that affect heparin affinity. Mutations of Lys114 or Lys125 in a higher heparin affinity antithrombin glycoform lacking the carbohydrate chain at Asn135 failed to block antiangiogenic activity despite the demonstrated effects of these mutations on heparin affinity in cleaved antithrombin. This is likely to reflect the significant residual heparin affinities of the mutant recombinant antithrombins relative to antiangiogenically active plasma forms of cleaved and latent antithrombins that contain the Asn135 carbohydrate chain (Table 1). Of particular interest, mutation of Lys114 in a glycoform lacking carbohydrate at Asn135 but also fucosylated on the Asn155-linked carbohydrate chain was able to block the antiangiogenic activity of cleaved antithrombin despite only a slightly lower heparin affinity than the unfucosylated variant. Fucosylation alone did not affect the antiangiogenic activity of the cleaved wild-type serpin, indicating that the heparin binding defect produced by the Lys114 mutation was required to block the activity. The Asn155-linked carbohydrate chain modestly interferes with heparin binding to wild-type antithrombin due to its proximity to the heparin-binding site and fucosylation is thought to enhance this interference by rigidifying the chain.26,27 Fucosylation could magnify the effect of the Lys114 mutation by blocking the ability of antiangiogenic antithrombin to simultaneously bind an antiangiogenic receptor and a heparan sulfate coreceptor. Alternatively, fucosylation may have greater effects on antiangiogenic antithrombin binding to a specific heparan sulfate domain than those observed for binding to heparin-agarose. That antiangiogenic forms of antithrombin have a different specificity for binding particular sequences in heparin than the anticoagulant native form of antithrombin is suggested by our finding that the heparin binding residues, Lys125 and Lys114, and the Asn135- and fucosylated Asn155-linked carbohydrate chains make very different contributions to binding heparin in cleaved compared with native antithrombins. Whether antithrombin antiangiogenic activity is mediated by specific heparan sulfate domains, such as those demonstrated to mediate the antiangiogenic activity of endostatin,48 will need to be addressed in future studies.
Most surprising was our finding that the native form of the Lys114 antithrombin mutant had acquired antiangiogenic activity without the need for the conformational change induced by reactive loop cleavage. Lys114 functions as a pivotal determinant of the specificity of native antithrombin for the anticoagulant heparin pentasaccharide sequence and the ability of heparin to conformationally activate the serpin.8,22 Mutation of Lys114 blocks conformational activation as evidenced by the inability of heparin to induce the high-affinity interaction associated with this activation (Table 1). This locks antithrombin in the native low-heparin affinity conformation in which the reactive loop is partially inserted in sheet A.8 The resemblance of this reactive loop inserted conformation of native antithrombin to the fully inserted reactive loop conformations of cleaved and latent conformations implies that insertion of the reactive loop into sheet A, whether it be partial or complete, and the structural changes associated with this insertion represent the critical conformational determinants of antiangiogenic activity. According to this idea, heparin activation of antithrombin causes the loss of these determinants by expelling the loop from sheet A and thus represents a conformational switch that is responsible for turning off antiangiogenic activity. Even the binding of native antithrombin to nonanticoagulant heparan sulfate sequences39 would be expected to generate the antiangiogenically inactive reactive loop expelled conformation, since low-affinity heparin induces nearly complete conformational activation of native antithrombin.49 Reactive loop insertion into sheet A may be required to express an antiangiogenic epitope on antithrombin that mediates its binding to an endothelial cell receptor and that becomes cryptic when the reactive loop is expelled.
The mechanism by which heparin promotes antithrombin antiangiogenic activity was not addressed by our studies. Since the antiangiogenic action of antithrombin involved in all cases the blocking of bFGF-dependent proangiogenic activities, one possible mechanism is that antithrombin binds to and blocks heparan sulfate chains that act as essential coreceptors to promote the dimerization and formation of a signaling-competent bFGF-FGF receptor complex.41,44,50 Contrasting this mechanism, our previous findings suggested an alternative indirect mechanism through which antiangiogenic antithrombins may inhibit bFGF proangiogenic functions.20 Antiangiogenic antithrombins were thus found to alter the expression of several genes in both growth factor–stimulated and unstimulated HUVECs. In particular, perlecan expression was found to be significantly down-regulated by antiangiogenic anti-thrombins independent of growth factor stimulation, suggesting that the antithrombins interact with an endothelial cell receptor to mediate such effects. Antithrombin signaling through its own receptor could therefore account for its ability to inhibit bFGF-induced proangiogenic activities through decreased expression of the perlecan coreceptor for bFGF.44,45 Our present findings would further suggest that antithrombin binding to its putative receptor requires its own heparan sulfate proteoglycan coreceptor. Such an indirect mechanism mediates the heparan sulfate–dependent antiangiogenic action of endostatin based on the findings that endostatin produces global changes in endothelial cell gene expression involving the down-regulation of proangiogenic genes and up-regulation of antiangiogenic genes,51 and these effects depend on heparan sulfate structures distinct from those that promote bFGF-receptor binding.48 Future work will be required to distinguish between such direct and indirect mechanisms for mediating antithrombin's antiangiogenic activity and for elucidating the role of heparan sulfate in this mechanism.
Prepublished online as Blood First Edition Paper, May 19, 2005; DOI 10.1182/blood-2005-02-0547.
Supported by a grant from the National Institutes of Health (HL-39888) to S.T.O. and by funds from the Vice Chancellor of Research, University of Illinois at Chicago awarded to W.Z.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Maurice Petitou of Sanofi Recherche (Toulouse, France) for generously providing the natural and high-affinity pentasaccharides and for critiquing the paper. We also thank Dr Peter Gettins (University of Illinois at Chicago) for providing helpful comments on the paper.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal