Abstract
Tissue factor (TF) circulates in plasma, largely on monocyte/macrophage-derived microvesicles that can bind activated platelets through a mechanism involving P-selectin glycoprotein ligand-1 (PSGL-1) on the microvesicles and P-selectin on the platelets. We found these microvesicles to be selectively enriched in both TF and PSGL-1, and deficient in CD45, suggesting that they arise from distinct membrane microdomains. We investigated the possibility that microvesicles arise from cholesterol-rich lipid rafts and found that both TF and PSGL-1, but not CD45, localize to lipid rafts in blood monocytes and in the monocytic cell line THP-1. Consistent with a raft origin of TF-bearing microvesicles, their shedding was significantly reduced with depletion of membrane cholesterol. We also evaluated the interaction between TF-bearing microvesicles and platelets. Microvesicles bound only activated platelets, and required PSGL-1 to do so. The microvesicles not only bound the activated platelets, they fused with them, transferring both proteins and lipid to the platelet membrane. Fusion was blocked by either annexin V or an antibody to PSGL-1 and had an important functional consequence: increasing the proteolytic activity of the TF-VIIa complex. These findings suggest a mechanism by which all of the membrane-bound reactions of the coagulation system can be localized to the surface of activated platelets.
Introduction
Injury to the blood vessel wall triggers a hemostatic response initiated by the adhesion and aggregation of platelets and amplified by both release of agonists from platelet granules and by production of thrombin by the soluble coagulation system. Thrombin potently activates platelets and stabilizes the thrombus by catalyzing the formation of insoluble fibrin polymers. Blood coagulation is initiated at the site of vessel injury by tissue factor (TF), a type I transmembrane protein that serves as a cofactor for the serine protease factor (F) VIIa in converting the zymogen FX to its active form FXa.1 FXa, in complex with FVa, then converts prothrombin to thrombin on a surface of anionic phospholipid provided by the activated platelet.2
For many years TF has been thought to reside almost exclusively on extravascular cells, forming a “hemostatic envelope” surrounding the vasculature.3 This conceptual framework fails to explain how coagulation is initiated within the vasculature in the absence of vessel wall injury or even when the injury is superficial, given that the subsequent reactions in the coagulation cascade are carried out most efficiently on the surface of activated platelets.2 If the blood must encounter TF outside of the blood vessel, propagation of the thrombus to the vascular space would require that the product of the TF/FVIIa complex, FXa, diffuse upstream through a sea of serine protease inhibitors to reach the activated platelet. A potential solution to this quandary has recently surfaced with the demonstration that TF also circulates in the blood under normal conditions,4 both associated with cell-derived membrane microvesicles5 and as a soluble, alternatively spliced form.6 TF-bearing microvesicles are believed to arise from cells of the monocyte/macrophage lineage, given that they express several surface proteins specific for these cells.7 They have also been postulated to have a hemostatic role, as they have been found to associate with experimentally induced platelet-rich thrombi,7,8 and to shorten clotting times in hemophilic mice.8 The microvesicles bind activated platelets in thrombi through a molecular bridge between P-selectin glycoprotein ligand-1 (PSGL-1) on microvesicles and P-selectin on the platelets.7 Failure of this hemostatic mechanism may explain the ability of agents that block the PSGL-1–P-selectin interaction to significantly inhibit experimental thrombosis.9-11
To gain insight into the role of TF-bearing microvesicles in initiating coagulation, we investigated both their subcellular origin and the nature of their interaction with activated platelets. We found that the microvesicles arise from regions of the monocyte/macrophage membrane rich in lipid rafts and are themselves rich in TF and PSGL-1—which we show localize to lipid rafts—and are relatively devoid of proteins excluded from rafts. TF-bearing microvesicles are thus similar to several enveloped viruses, including HIV-112 and influenza virus,13 which arise from membrane rafts and infect cells by fusing with the target cell membranes. We therefore investigated the possibility that TF-bearing microvesicles fuse with activated platelets and found that they do, in the process transferring their complement of membrane proteins, including TF. These results provide a mechanism by which blood coagulation can be initiated and propagated on a single cell surface, that of the activated platelet, and, in the physiologic setting, restricted to the site of vessel injury.
Materials and methods
Isolation of platelets and monocytes
Blood was drawn from healthy volunteers who consented to the procedure under a protocol approved by the institutional review board of Baylor College of Medicine. Washed platelets were prepared as described,14 and were resuspended in Tyrode buffer containing 2 mM CaCl2.
Blood mononuclear cells were isolated from fresh buffy coats obtained from a blood bank (Gulf Coast Regional Blood Center, Houston, TX), using a single density barrier of lymphoprep (Accurate, Westbury, NY). Monocytes were separated from lymphocytes by allowing the monocytes to adhere to a cell-culture dish. The monocytes were then detached using EDTA (ethylenediaminetetraacetic acid), washed twice, and resuspended in tris-buffered saline (TBS) containing 2 mM CaCl2. Monocytes made up over 95% of the cells in the preparation, as determined by Wright staining.
Microvesicle preparation
Tissue factor–bearing microvesicles were generated from either 1 × 107 blood-derived monocytes or 1 × 107 THP-1 cells (American Type Culture Collection, Manassas, VA), a human monocytoid cell line. Tissue factor expression was induced with lipopolysaccharide (LPS; 1 μg/mL; Sigma-Aldrich, St Louis, MO) for 6 hours at 37°C. The cells were induced to microvesiculate by stimulating them either with 1 μg/mL LPS alone for 6 hours, or with 5 μM calcium ionophore A23 187 (Calbiochem, Oakland, CA) for 15 minutes, after the 6-hour LPS treatment. Microvesicles were isolated from the cells by centrifuging the suspension at 5000g for 5 minutes, and collecting the upper half of the supernatant. Careful microscopic inspection confirmed the lack of intact cells in the microvesicle-containing supernatant. Microvesicle suspensions (1 mL) were washed twice in 10 mL TBS by centrifugation/resuspension (200 000g, 4 hours). The final pellet was resuspended in 1 mL TBS.
Plasma microvesicles were isolated from 24 mL of cell-free, acid-citrate-dextrose (ACD)–anticoagulated plasma prepared within 20 minutes of drawing blood. Cell-free plasma was obtained by 3 sequential centrifugation steps: platelet-rich plasma (PRP) was first obtained by centrifuging blood at 700g for 6 minutes; platelet-poor plasma (PPP) was then obtained by centrifuging PRP at 1500g for 7 minutes; and cell-free plasma was obtained by spinning PPP at 5000g for 5 minutes. Microvesicles were sedimented by centrifuging the plasma at 200 000g for 4 hours at 4°C, then resuspended in 0.5 mL TBS. Tissue factor–bearing microvesicles were isolated from this suspension by capturing them with polystyrene beads (Polysciences, Warrington, PA) coated with a murine anti–human TF monoclonal antibody prepared by immunization with a peptide corresponding to amino acids 1 through 25 of human TF (no. 4508; American Diagnostica, Stamford, CT). Beads coated with nonimmune mouse immunoglobulin G (IgG) did not capture a significant number of microvesicles (data not shown).
To examine the possibility that microvesicles transfer TF and PSGL-1 to platelets, we incubated monocyte or THP-1 microvesicles with either unactivated platelets or platelets activated with 5 μg/mL type I collagen (Helena, Beaumont, TX) or 5 μM A23187 in the presence of either nonspecific mouse IgG or the blocking monoclonal PSGL-1 antibody, KPL-1 (a generous gift from Karen R. Snapp, University of Illinois). Samples were incubated with shaking for 45 to 60 minutes at 37°C. Unbound microvesicles were removed by differential centrifugation, spinning the samples at 2000g for 5 minutes, performed 5 times.
Immunoblotting
Samples were subjected to standard sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as described.14 The following antibodies were used for immunoblotting: KPL-1; a polyclonal rabbit antibody against human tissue factor (generously provided by Usha Pendurthi, University of Texas); the human CD45 monoclonal antibody 135-4C5 (Chemicon, Temecula, CA); and an anti–flotillin-1 monoclonal antibody (clone 18; BD Biosciences, San Jose, CA). Densitometric analysis was performed with Scion image software (Frederick, MD).
Lipid raft isolation
Approximately 1 × 107 THP-1 cells were lysed by the addition of 1 mL ice-cold 1% Triton X-100 in MES (2-(N-morpholino)ethanesulfonic acid)–buffered saline (25 mM MES, pH 6.5, 150 mM NaCl) containing protease inhibitors (Roche complete mini tablets; Roche Indianapolis, IN) and phosphatase inhibitors and incubating for 30 minutes at 4°C. After the lysate was clarified by centrifugation at 2000g for 5 minutes, an equal volume of 80% sucrose was added, and the sample was then transferred to an ultracentrifuge tube and sequentially overlaid with 5.5 mL and 4.5 mL of 30% and 5% sucrose solutions, respectively, at 4°C. The samples were then centrifuged at 200 000g for 18 to 20 hours at 4°C, using an SW40 rotor (Beckman, Fullerton, CA). Eleven equal fractions were collected from top to bottom. Proteins from each sample were precipitated with trichloroacetic acid (10% vol/vol), then resuspended in 100 μL SDS sample buffer, and subjected to SDS-PAGE and immunoblotting. In some experiments, the cells were incubated in 5 mM methyl-β-cyclodextrin (MβCD) (Sigma-Aldrich, St Louis, MO) for 30 minutes at 37°C or in control buffer, then washed and fractionated on the sucrose density gradient.
Flow cytometry
The acquisition of TF or PSGL-1 by platelets incubated with monocyte microvesicles was assessed by flow cytometry using an EPICS XL flow cytometer (Beckman-Coulter, Fullerton, CA). Experiments were performed in the presence or absence of annexin V (100 μg/mL). The samples were handled as described under “Microvesicle preparation,” and were then probed with fluorescently conjugated antibodies directed against either TF or PSGL-1 (Becton Dickinson, San Jose, CA). Baseline fluorescence was determined using isotype-matched fluorescent antibodies.
To determine the effect of cholesterol depletion on the generation of microvesicles, THP-1 cells were treated with either 5 mM MβCD or control buffer for 30 minutes at 37°C. The cells were washed twice and induced to microvesiculate with 2 μM A23187 for 15 minutes at 37°C. Microvesicles were isolated and labeled with fluorescein isothiocyanate (FITC)–conjugated annexin V. The microvesicles were then diluted 20-fold in TBS, 2 mM CaCl2, and counted as the number of FITC-positive events during a 300-second period, setting the flow cytometer to acquire events using logarithmic gains for both forward scatter (FS) and side scatter (SS). More than 95% of the total events were positive for FITC–annexin V. We ascertained that we were indeed counting microvesicles as we observed no microvesicles in the microvesicle buffer alone and were able to completely eliminate events by filtering the suspension through a 0.2-μm filter.
Membrane fusion assay
Membrane fusion was studied with a lipid-mixing assay based on the one developed by Peyron et al.15 Briefly, 1 × 107 THP-1 cells suspended in 1 mL TBS with 2 mM CaCl2 were labeled by incubating them with 302 nmol of lipid micelles containing N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (NBD-PE), rhodamine-phosphatidylethanolamine (Rh-PE; Molecular Probes, Eugene, OR), and the cationic phospholipid, dioleoyl-3-trimethylammonium-propane (DOTAP; Avanti Polar Lipids, Alabaster, AL) in a 2:2:1 molar ratio, respectively, for 15 minutes at 4°C. The labeled cells were then washed twice in 30 mL TBS and finally resuspended in 1 mL TBS with 2 mM CaCl2. Microvesiculation was subsequently induced with 5 μM A23187 at 37°C, and the microvesicles were isolated as described in “Microvesicle preparation.”
The labeled microvesicles were incubated with either unactivated platelets or collagen-activated platelets suspended in buffer. Samples were continuously shaken at 37°C in the presence or absence of either KPL-1 (50 μg/mL), nonspecific mouse IgG (50 μg/mL), or annexin V (100 μg/mL). At the indicated time points, NBD fluorescence (530 nm) was read in a fluorometer (Molecular Devices, Sunnyvale, CA) at an excitation wavelength of 470 nm. Aside from the NBD-PE and Rh-PE, none of the reagents used in the lipid-mixing experiments had significant autofluorescence or fluorescence-quenching properties.
Fluorescence microscopy
To directly visualize the transfer of lipid from microvesicles to platelets, we labeled THP-1 cells with the fluorescent lipid octadecylrhodamine (R18; Molecular Probes), using a method based on the one described by Morris et al.16 Briefly, 30 μL of R18 (1 mg/mL in ethanol) was added to 1 × 107 THP-1 cells suspended in 10 mL TBS 2 mM CaCl2. Cells were incubated for 15 minutes at room temperature with constant shaking. RPMI-1640 medium (40 mL) with 10% fetal bovine serum was then added to the cells to quench all the unbound R18. After 20 minutes, the cells were washed 3 times with TBS and resuspended in 1 mL TBS with 2 mM CaCl2. The cells were then induced to microvesiculate and the microvesicles isolated. The suspension of labeled microvesicles (100 μL) was then incubated for 60 minutes at 37°C over a monolayer of activated platelets spread over immobilized fibrinogen on a glass coverslip. This monolayer was prepared by plating 2 × 104 platelets in 300 μL Tyrode buffer over a fibrinogen-coated (100 μg/mL) glass coverslip for 60 minutes at 37°C. After this incubation, all unbound microvesicles were aspirated and removed by washing the coverslip 4 times with TBS. The coverslips were then mounted on a glass slide with a drop of Fluoromount G (Electron Microscopy Sciences, Hatfield, PA), and observed immediately under an upright fluorescent microscope using the rhodamine channel.
TF-VIIa activity
TF-VIIa activity was measured using a chromogenic assay that relies on the ability of TF-VIIa to activate FX to FXa. Washed TF-bearing microvesicles were obtained from 5 × 107 LPS-stimulated THP-1 cells, and incubated for 45 minutes with 100 μg/mL of either unlabeled annexin V or bovine serum albumin (BSA), then washed 3 times in 10 mL TBS 2 mM CaCl2 by sedimentation/resuspension (centrifugation at 200 000g for 4 hours). They were then resuspended in 1 mL TBS, 2 mM CaCl2. We determined that these microvesicles had been “cloaked” with unlabeled annexin V by showing that they failed to bind FITC–annexin V. The annexin V– or BSA-treated microvesicles were then incubated with 2 × 107 washed platelets suspended in Tyrode buffer, 2 mM CaCl2, for 30 minutes at 37°C with continuous shaking. In some experiments, platelets were activated with 5 μg/mL collagen, or microvesicles were pretreated with 50 μg/mL of either KPL-1 or mouse IgG. Unbound microvesicles were then removed by sedimenting the platelets at 1150g for 10 minutes. Platelets were then resuspended in 0.5 mL TBS 5 mM CaCl2, supplemented with 150 nM FX, and 10 nM FVIIa (American Diagnostica), and incubated at 37°C for 30 minutes. The microvesicle/platelet mixture was then filtered through a 0.2-μm filter, and 0.5 mM Spectrozyme-Xa (American Diagnostica) was added to 0.3 mL of the filtrate. After 60 minutes at 37°C, absorbance at 405 nm was recorded in a microplate reader (Microplate Devices, Menlo Park, CA). As controls, TF-VIIa activity was measured in samples in which FVIIa, FX and/or spectrozyme-Xa were omitted from the reaction. All samples were run in duplicate.
Statistics
Results are expressed as mean values plus or minus the standard deviation (SD), and analyzed using either a paired t test or analysis of variance (ANOVA), depending on the nature of the data. A P value equal to .05 was considered statistically significant.
Results
Monocyte microvesicles are selectively enriched in TF and PSGL-1 and relatively devoid of CD45
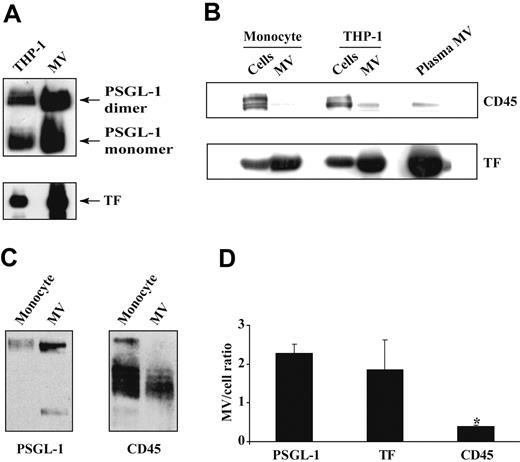
Tissue factor–bearing microvesicles were generated from LPS-stimulated monocytes or THP-1 cells by treating the cells with either LPS alone or LPS plus calcium ionophore. Regardless of how they were generated, all microvesicle preparations contained much greater quantities of TF and PSGL-1 than their cells of origin, a difference that persisted even if the entire cell and microvesicle samples had been equally diluted, or if they had been adjusted for a similar protein content (Figure 1A). These methods of normalization, however, left open the possibility that the observed difference in TF and PSGL-1 content in the microvesicles compared with the cells resulted from the greater membrane-volume ratio of the microvesicle pellet. We therefore probed for another monocyte membrane protein, the phosphatase CD45. In marked contrast to both TF and PSGL-1, CD45 was present in much greater amounts in the cells than in their microvesicles (Figure 1B-C). By densitometry, the microvesicle-cell ratios for TF and PSGL-1 were 2.2 and 1.9, respectively, whereas that of CD45 was only 0.4 (Figure 1D). Similar ratios were obtained for monocytes and TF-bearing microvesicles isolated from the plasma of healthy individuals (Figure 1B). These findings suggested that the microvesicles do not arise randomly from the monocyte plasma membrane but instead arise from selected membrane regions of distinct composition.
PSGL-1 and TF, but not CD45, localize to lipid raft fractions
Of interest, in other cells both TF17 and PSGL-118 have been localized to specialized cholesterol-rich membrane microdomains known as lipid rafts,19 domains from which CD45 has also been shown to be excluded.20 We therefore determined the relative distribution of the 3 proteins within raft and nonraft fractions in monocytes and THP-1 cells. We examined PSGL-1 and CD45 in both cell types, and TF in LPS-stimulated monocytes (monocytes do not constitutively express TF). Significant portions of both PSGL-1 and TF localized to triton-insoluble fractions (fractions 5-7), where the raft-resident protein flotillin-121 also localized (Figure 2A-B). By contrast, CD45 was completely excluded from these fractions (Figure 2C).
Depletion of membrane cholesterol with MβCD, which disrupts rafts,19 resulted in the complete relocation of PSGL-1, TF, and flotillin-1 from the raft fractions to the bottom fractions (10 and 11) of the gradient (Figure 2D). MβCD did not alter PSGL-1 or TF surface expression, as assessed by flow cytometry (Figure 2E).
Content of PSGL-1, TF, and CD45 in monocytes and their microvesicles (MVs). THP-1 cells or blood monocytes were stimulated with LPS; microvesiculation was induced with calcium ionophore. Cells were separated from their shed MVs by sedimenting the cells at 5000g for 5 minutes. The entire cell pellet and MV suspension were resuspended and diluted to the same extent, and lysed in SDS sample buffer. Equal volumes of the lysates were subjected to SDS-PAGE and analyzed by Western blot. (A) PSGL-1 and TF content in THP-1 cells and their shed MVs. (B) TF and CD45 content in monocytes, THP-1 cells, their respective MVs, and in MVs isolated from fresh plasma. (C) PSGL-1 and CD45 content in monocytes and their shed MVs. (D) Densitometric analysis of the MV/cell ratio of PSGL-1, TF, and CD45. These data are represented as the mean value for the MV/cell ratio in 6 different experiments plus or minus SD. *P < .003 CD45 versus PSGL-1 and versus TF.
Content of PSGL-1, TF, and CD45 in monocytes and their microvesicles (MVs). THP-1 cells or blood monocytes were stimulated with LPS; microvesiculation was induced with calcium ionophore. Cells were separated from their shed MVs by sedimenting the cells at 5000g for 5 minutes. The entire cell pellet and MV suspension were resuspended and diluted to the same extent, and lysed in SDS sample buffer. Equal volumes of the lysates were subjected to SDS-PAGE and analyzed by Western blot. (A) PSGL-1 and TF content in THP-1 cells and their shed MVs. (B) TF and CD45 content in monocytes, THP-1 cells, their respective MVs, and in MVs isolated from fresh plasma. (C) PSGL-1 and CD45 content in monocytes and their shed MVs. (D) Densitometric analysis of the MV/cell ratio of PSGL-1, TF, and CD45. These data are represented as the mean value for the MV/cell ratio in 6 different experiments plus or minus SD. *P < .003 CD45 versus PSGL-1 and versus TF.
Isolation of lipid rafts from monocytes. Triton X-100 lysates from monocytes were fractionated by centrifugation over a discontinuous sucrose gradient. Eleven equal fractions were obtained and assessed by Western blotting for the presence of (A) PSGL-1, (B) TF, and (C) CD45. Lipid rafts were identified by the presence of the raft-marker, flotillin-1. (D) Effect of membrane cholesterol depletion with MβCD on the localization of PSGL-1 and TF to lipid rafts. (E) As assessed by flow cytometry, cholesterol depletion did not affect the surface expression of PSGL-1 (top panels) or TF (bottom panels) on LPS-treated THP-1 cells. Background fluorescence was set with a fluorescent mouse IgG control (empty histograms). (F) Effect of membrane cholesterol depletion with 5 mM MβCD on the generation of microvesicles induced by calcium ionophore in THP-1 cells. The results shown are representative of 3 separate experiments. Values are shown as mean plus or minus SD. *P = .04 (n = 4).
Isolation of lipid rafts from monocytes. Triton X-100 lysates from monocytes were fractionated by centrifugation over a discontinuous sucrose gradient. Eleven equal fractions were obtained and assessed by Western blotting for the presence of (A) PSGL-1, (B) TF, and (C) CD45. Lipid rafts were identified by the presence of the raft-marker, flotillin-1. (D) Effect of membrane cholesterol depletion with MβCD on the localization of PSGL-1 and TF to lipid rafts. (E) As assessed by flow cytometry, cholesterol depletion did not affect the surface expression of PSGL-1 (top panels) or TF (bottom panels) on LPS-treated THP-1 cells. Background fluorescence was set with a fluorescent mouse IgG control (empty histograms). (F) Effect of membrane cholesterol depletion with 5 mM MβCD on the generation of microvesicles induced by calcium ionophore in THP-1 cells. The results shown are representative of 3 separate experiments. Values are shown as mean plus or minus SD. *P = .04 (n = 4).
Cholesterol depletion decreases microvesicle generation
If lipid rafts have an important role in the generation of microvesicles, then depletion of membrane cholesterol should decrease microvesicle production. Accordingly, treatment with 5 mM MβCD reduced ionophore-stimulated shedding of microvesicles from THP-1 cells by approximately 40% (Figure 2F). We used calcium ionophore because it stimulates cells independent of receptor activation; the actions of other agonists (eg, LPS22 ) could be affected by cholesterol depletion because the initial signaling pathways may depend on the localization of their receptors to rafts.
Activated platelets acquire TF and PSGL-1 when incubated with monocyte microvesicles
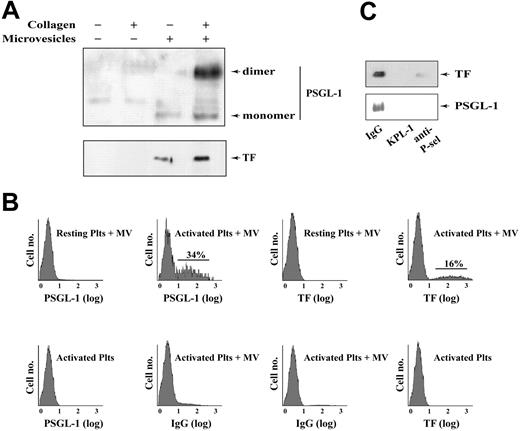
We next examined the association of TF-bearing microvesicles with activated platelets. Neither quiescent nor activated platelets contained detectable quantities of TF or PSGL-1. We incubated TF-bearing microvesicles from LPS-treated THP-1 cells with either resting or collagen-activated platelets and removed the unbound microvesicles by differential centrifugation. Activated platelets acquired a substantial quantity of TF and PSGL-1; unactivated platelets acquired only a minor amount (Figure 3A). By flow cytometry, on average 27% and 17% of activated platelets acquired PSGL-1 and TF, respectively, when incubated with monocyte microvesicles (Figure 3B). Transfer of the 2 proteins was inhibited almost completely by antibodies against PSGL-1 or P-selectin (Figure 3C), confirming previous studies indicating that the interaction between platelets and monocyte microvesicles is dependent on P-selectin and PSGL-1.7,23
Monocyte microvesicles fuse with activated platelets
These studies demonstrate an interaction between microvesicles and activated platelets but do not distinguish passive attachment from the fusion of their membranes. The fusion of microvesicles with the target platelet membrane is a possibility suggested by the high expression of the fusogenic phospholipids phosphatidylserine and phosphatidylethanolamine on both membranes. To investigate whether the monocyte-derived microvesicles are able to fuse with platelets, we used a lipid-mixing assay that relies on the interactions between 2 fluorescently labeled phospholipids (N-NBD-phosphatidylethanolamine [PE] and N-rhodamine [Rh]-PE) incorporated into the microvesicles.15 When the 2 probes are in close proximity, as in the labeled microvesicles, light emitted from excited NBD-PE (λ 530 nm) excites Rh-PE to emit light at 585 nm. When the membrane carrying the 2 fluorophores fuses with an unlabeled membrane, the fluorophores are diluted and the signal emitted by Rh decreases while the NBD signal (530 nm) increases.15 Membrane fusion can thus be followed by monitoring the increase in NBD fluorescence as a function of time.
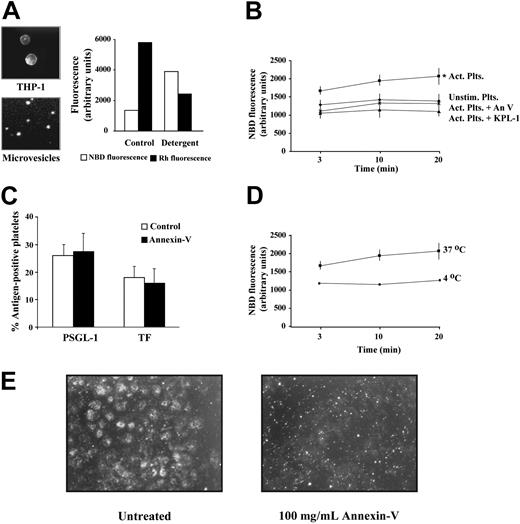
THP-1 cells labeled with both fluorescent lipids produced microvesicles that retained both probes (Figure 4A). To determine if the ratio of the 2 fluorophores in the microvesicle membranes was adequate for the lipid-mixing assays, we simulated membrane fusion by solubilizing the labeled microvesicles with detergent (1% SDS). As expected, dilution of the probes in the detergent-treated microvesicles caused a marked increase in NBD fluorescence and a concomitant decrease in Rh fluorescence (Figure 4A), indicating that the labeling was adequate for the membrane fusion assays. We incubated labeled microvesicles with either unstimulated or activated platelets. A relative increase in NBD fluorescence in the activated platelets compared with the unstimulated platelets was apparent at the earliest assay time (3 minutes). By 20 minutes the NBD fluorescence was 3.7-fold greater in the activated platelet suspension, indicating that the microvesicles had fused with the activated platelets (Figure 4B). KPL-1, the PSGL-1 antibody, markedly inhibited fusion, yielding fluorescence values that probably represent the true baseline, in which the microvesicles do not fuse with the platelets.
Monocyte microvesicles transfer TF and PSGL-1 to activated platelets via PSGL-1. (A) Collagen-activated or unstimulated washed platelets were incubated with either monocyte microvesicles or control buffer. Platelets were sedimented and unbound microvesicles were removed by washing 5 times. The platelets were pelleted, lysed in SDS sample buffer, and analyzed by SDS-PAGE and Western blotting for PSGL-1 and TF. (B) Flow cytometric analysis of platelets incubated with either microvesicles or buffer, handled as described in (A). Samples using resting platelets plus microvesicles, activated platelets alone, and samples probed with control fluorescent IgG served as the negative controls in these experiments. (C) Treatment with anti–PSGL-1 (KPL-1) or an anti–P-selectin antibody, but not a nonspecific mouse IgG, blocked the transfer of TF and PSGL-1.
Monocyte microvesicles transfer TF and PSGL-1 to activated platelets via PSGL-1. (A) Collagen-activated or unstimulated washed platelets were incubated with either monocyte microvesicles or control buffer. Platelets were sedimented and unbound microvesicles were removed by washing 5 times. The platelets were pelleted, lysed in SDS sample buffer, and analyzed by SDS-PAGE and Western blotting for PSGL-1 and TF. (B) Flow cytometric analysis of platelets incubated with either microvesicles or buffer, handled as described in (A). Samples using resting platelets plus microvesicles, activated platelets alone, and samples probed with control fluorescent IgG served as the negative controls in these experiments. (C) Treatment with anti–PSGL-1 (KPL-1) or an anti–P-selectin antibody, but not a nonspecific mouse IgG, blocked the transfer of TF and PSGL-1.
Microvesicles fuse with activated platelets. (A) Fluorescence microscopy (NBD channel) of THP-1 cells labeled with NBD-PE and Rh-PE (left panel, top) and of their shed microvesicles (left panel, bottom). Original magnifications, × 40 and × 400 on a Nikon Eclipse EP00 upright microscope equipped with 4 ×/0.13 NA and 40 ×/0.75 NA objective lenses (Nikon, Melville, NY), respectively. Images were captured using a Photometrics CoolSnap CS digital camera (Photometrics, Tucson, AZ). Solubilization of the labeled microvesicles with 1% SDS diluted the probes, resulted in an increase in NBD fluorescence, and a decrease in Rh fluorescence (right panel). (B) Labeled microvesicles were incubated with either: (x) unstimulated platelets; (▪) activated platelets; (♦) activated platelets + annexin V (100 μg/mL); (▴) activated platelets + anti–PSGL-1 antibody, KPL-1 (5 μg/mL). (C) Transfer of PSGL-1 and TF to activated platelets by monocyte microvesicles, as assessed by flow cytometry. Annexin V (100 μg/mL) did not affect the number of platelets that acquire these proteins. Background fluorescence was determined using fluorescent nonspecific mouse IgG. n = 3. (D) Membrane fusion between activated platelets and THP-1 microvesicles at 37°C (▪), and at 4°C (▪). (E) Fluorescence microscopy in rhodamine channel (×40) showing the transfer of the fluorescent lipid R18 from labeled microvesicles to platelets adhered on immobilized fibrinogen. Transfer of the lipid resulted in the spread platelets becoming fluorescent, an effect that was blocked by annexin V. The small fluorescent spots in the annexin V–treated sample correspond to the labeled microvesicles. These images are representative of 4 experiments. Act indicates activated; Plts, platelets; Unstim, unstimulated; An V, annexin V. Error bars indicate ± standard deviation (SD) in panels B-D.
Microvesicles fuse with activated platelets. (A) Fluorescence microscopy (NBD channel) of THP-1 cells labeled with NBD-PE and Rh-PE (left panel, top) and of their shed microvesicles (left panel, bottom). Original magnifications, × 40 and × 400 on a Nikon Eclipse EP00 upright microscope equipped with 4 ×/0.13 NA and 40 ×/0.75 NA objective lenses (Nikon, Melville, NY), respectively. Images were captured using a Photometrics CoolSnap CS digital camera (Photometrics, Tucson, AZ). Solubilization of the labeled microvesicles with 1% SDS diluted the probes, resulted in an increase in NBD fluorescence, and a decrease in Rh fluorescence (right panel). (B) Labeled microvesicles were incubated with either: (x) unstimulated platelets; (▪) activated platelets; (♦) activated platelets + annexin V (100 μg/mL); (▴) activated platelets + anti–PSGL-1 antibody, KPL-1 (5 μg/mL). (C) Transfer of PSGL-1 and TF to activated platelets by monocyte microvesicles, as assessed by flow cytometry. Annexin V (100 μg/mL) did not affect the number of platelets that acquire these proteins. Background fluorescence was determined using fluorescent nonspecific mouse IgG. n = 3. (D) Membrane fusion between activated platelets and THP-1 microvesicles at 37°C (▪), and at 4°C (▪). (E) Fluorescence microscopy in rhodamine channel (×40) showing the transfer of the fluorescent lipid R18 from labeled microvesicles to platelets adhered on immobilized fibrinogen. Transfer of the lipid resulted in the spread platelets becoming fluorescent, an effect that was blocked by annexin V. The small fluorescent spots in the annexin V–treated sample correspond to the labeled microvesicles. These images are representative of 4 experiments. Act indicates activated; Plts, platelets; Unstim, unstimulated; An V, annexin V. Error bars indicate ± standard deviation (SD) in panels B-D.
Effect of membrane fusion on TF-VIIa activity. (A) TF-bearing microvesicles were incubated with 100 μg/mL of either bovine serum albumin (BSA) or unlabeled annexin V, and then washed. Microvesicles (MVs) treated with annexin V exhibited no binding of FITC-conjugated annexin V above an EDTA-treated sample, indicating blockade of phosphatidylserine on their surfaces. (B) TF-VIIa activity was measured using a chromogenic assay based on Xa generation. These values are adjusted for the direct effect of annexin V on the chromogenic assay. (C) Annexin V–coated microvesicles alone had approximately 35% less TF-VIIa activity than an equal number of BSA-treated microvesicles. n = 4; *P < .05. Error bars indicate ± SD.
Effect of membrane fusion on TF-VIIa activity. (A) TF-bearing microvesicles were incubated with 100 μg/mL of either bovine serum albumin (BSA) or unlabeled annexin V, and then washed. Microvesicles (MVs) treated with annexin V exhibited no binding of FITC-conjugated annexin V above an EDTA-treated sample, indicating blockade of phosphatidylserine on their surfaces. (B) TF-VIIa activity was measured using a chromogenic assay based on Xa generation. These values are adjusted for the direct effect of annexin V on the chromogenic assay. (C) Annexin V–coated microvesicles alone had approximately 35% less TF-VIIa activity than an equal number of BSA-treated microvesicles. n = 4; *P < .05. Error bars indicate ± SD.
Both monocyte microvesicles24 and collagen-activated platelets25 express surface phosphatidylserine, an anionic phospholipid that promotes the fusion of both synthetic and biologic membranes.26-28 We tested the potential role of phosphatidylserine in microvesicle fusion with platelets by using annexin V, a protein that binds phosphatidylserine with high affinity and specificity.29 Annexin V did not impair binding of the microvesicles to activated platelets (Figure 4C), but inhibited membrane fusion (Figure 4B).
Lipid transfer from microvesicles to adherent platelets
To directly visualize the transfer of lipid from the microvesicles to activated platelets, we incubated activated platelets adhered on immobilized fibrinogen with THP-1 microvesicles labeled with R18. Incubation of these microvesicles with the adherent platelets for 60 minutes at 37°C resulted in the acquisition of the fluorescent lipid by the spread platelets (Figure 4E). The transfer of this lipid from the microvesicles to the platelets was blocked by 100 μg/mL annexin V.
Fusion of monocyte-derived microvesicles with platelets increases TF-VIIa activity
Several studies have noted that fibrin generation in platelet thrombi correlates with the association of TF-bearing microvesicles with the thrombi.7,8 To examine whether fusion of the TF-bearing microvesicles with platelets increases TF-VIIa activity, we examined activity in microvesicle-platelet suspensions that were either untreated or incubated with annexin V, which allows the microvesicles to bind the platelets, but not to fuse. The microvesicles treated with annexin V were effectively cloaked, as they were unable to subsequently bind FITC–annexin V (Figure 5A). Washed platelets alone had negligible TF-VIIa activity. In contrast, platelets incubated with TF-bearing microvesicles exhibited a significant TF-VIIa activity, which increased substantially when the platelets were first activated with collagen (Figure 5B). The TF-VIIa activity in platelets incubated with annexin V–coated TF-bearing microvesicles was significantly less than in platelets incubated with bovine serum albumin (BSA)–treated TF-bearing microvesicles, even when adjusted for the membrane-fusion–independent effect of annexin V on the assay. We determined that annexin V–coated microvesicles had 35% less TF-VIIa activity than an equal number of microvesicles incubated with BSA instead of annexin V (Figure 5C). When fusion was prevented by annexin V, the TF-VIIa activity was only 45% of that in the mixture of control microvesicles and activated platelets. The TF-VIIa activity of the microvesicles was inhibited not only by annexin V, but also by KPL-1. Thus, the increase in coagulant efficiency seen when TF-bearing microvesicles associate with activated platelets requires both the binding and fusion of the microvesicles with the activated platelets. The specificity of the assay was confirmed using an anti-TF monoclonal antibody.
Discussion
Considerable evidence has surfaced that TF circulates in plasma, largely on microvesicles that can bind activated platelets through a mechanism involving PSGL-1 on the microvesicles and P-selectin on the platelets.7,8,23 Although there is good evidence that these microvesicles arise from monocytes or macrophages,7 their subcellular origin was unknown. We found monocyte microvesicles to contain 80% and 120% more TF and PSGL-1, respectively, than their cells of origin. In contrast, microvesicles contained approximately 60% less CD45 than the cells that shed them. Interestingly, TF-bearing microvesicles isolated from the plasma of healthy individuals exhibited a ratio of TF to CD45 similar to monocyte microvesicles generated in vitro, indicating that the 2 species of microvesicles are fundamentally similar. This peculiar protein-expression profile suggested to us that microvesicles arise from specific microdomains on the monocyte membrane. Indeed, our data indicate that monocyte-derived TF-bearing microvesicles arise from lipid rafts, or from regions of high raft content. In the cells of origin, both TF and PSGL-1 localized to lipid rafts, whereas CD45 was excluded from the raft fractions (Figure 2). These findings are consistent with studies showing that TF17 and PSGL-1,18 but not CD45,20 localize to lipid rafts in other cell types, and provide an explanation for the intense clustering of TF and PSGL-1 that Rauch and colleagues observed on the surface of microvesicles.23 As expected based on the known dependence of raft structure on membrane cholesterol content, disruption of lipid rafts by depleting cell membrane cholesterol decreased the number of shed microvesicles by approximately 40%. Our findings may provide insight into the origins of microvesicles in vivo and suggest a connection between hypercholesterolemia and the prothrombotic state, and may also help explain the antithrombotic effects of cholesterol-lowering therapies.30
The origin of TF-bearing microvesicles from lipid rafts is strikingly analogous to the budding of HIV and other enveloped viruses. Nguyen and Hildreth have shown that HIV virions originating from infected T lymphocytes incorporate raft-associated proteins on their envelopes, but lack the raft-excluded protein CD45, despite its abundance on the virion-shedding cells.12 These investigators also showed that depleting membrane cholesterol from infected cells markedly reduced viral budding.31 Another interesting similarity between monocyte microvesicles24 and HIV32 is that both express phosphatidylserine on their surfaces. In addition to playing an important role in coagulation33 and in the clearance of apoptotic bodies,34 phosphatidylserine promotes membrane fusion. Both phosphatidylserine and phosphatidylethanolamine have small headgroups and kinked acyl chains, forcing them into an upright cone shape that favors negative spontaneous curvature and membrane fusion.26-28 Consistent with these physicochemical properties, phosphatidylserine has been shown to mediate the membrane fusion events that lead to myotube formation35 and fertilization.36 Also, Callahan and coworkers recently showed that phosphatidylserine on the HIV envelope is critical for viral infectivity,32 strongly suggesting that it is necessary for HIV fusion with target cells. Similarly, both TF-bearing microvesicles24 and activated platelets37 express large amounts of phosphatidylserine on their surfaces. In the current studies, we show that TF-bearing microvesicles not only attach to activated platelets, but also fuse with them. Efficient fusion required platelet activation and was rapid, occurring within 3 minutes of addition of the microvesicles to the platelets. Fusion was preceded by docking of the microvesicles to the platelets through a PSGL-1–P-selectin interaction. The small amount of fusion observed when microvesicles were incubated with unstimulated platelets very likely resulted from a slight degree of activation of the platelets that occurred during their preparation. This interpretation is supported by the observation that an anti–PSGL-1 antibody reduced NBD fluorescence, the index of fusion, to levels below those observed in stimulated platelets (Figure 4B). In support of a critical role for phosphatidylserine in the fusion event, annexin V blocked fusion but did not interfere with the docking event. The final bit of evidence that the microvesicles are truly fusing with the platelets was the lack of a change in energy transfer when the incubation was performed at 4°C, a temperature known to preclude efficient membrane fusion.28 Although we only provide evidence that PSGL-1 and P-selectin are required for the binding of monocyte microvesicles to activated platelets, other adhesive interactions between microvesicles and platelets may contribute to their binding. Two notable candidate molecules on the monocyte microvesicle are l-selectin (Li et al, submitted) and the β2 integrin, MAC-1,38 both of which have been shown to bind platelet GPIbα. Further, because there is evidence that both l-selectin39 and MAC-121 localize to lipid rafts, they too may be enriched in monocyte microvesicles.
In our studies, platelets that were not incubated with monocyte microvesicles did not contain appreciable amounts of either TF or PSGL-1. Incubation of activated platelets with microvesicles, however, led to the acquisition of these 2 proteins by the platelets, with roughly 17% and 27% of platelets becoming positive for TF and PSGL-1, respectively. These results are similar to those obtained by Rauch and colleagues, who observed that 14% of activated platelets acquired TF when incubated with TF-bearing microvesicles.23 These findings may help explain reports that both TF40,41 and PSGL-142 are present on platelets, particularly in disease states where some platelet activation is expected. The demonstration of protein transfer by microvesicle fusion is not unprecedented, as Mack and colleagues showed that microvesicles expressing the chemokine receptor CCR5 could transfer this protein to cells lacking it, in the process rendering them prone to HIV infection.43 Although membrane fusion was not directly assessed in this study, the fact that the cells that became CCR5-positive also became susceptible to HIV infection strongly suggests that the microvesicles fused with the target cell membrane.
One phenomenon that we did not investigate in this study, but which we are currently pursuing, is whether monocyte microvesicles may also bind and fuse with platelet microvesicles released by the activated platelets. Like activated platelets, platelet microvesicles express P-selectin44 and phosphatidylserine,25,44 making their fusion with monocyte microvesicles a distinct possibility. If these 2 species of microvesicles do indeed fuse with each other, our reading of membrane fusion between monocyte microvesicles and activated platelets may be overestimated.
One question that arises immediately regarding the fusion event is whether it influences the activity of TF in initiating coagulation. TF-bearing microvesicles normally circulate in plasma, but do not effectively initiate coagulation. Obviously, a mechanism must exist to suppress TF-VIIa activity in the absence of vessel injury. This may involve soluble inhibitors in plasma, such as tissue factor pathway inhibitor (TFPI), or conformational regulation of TF so as to impede the binding of VIIa and thus initiate coagulation. A third mechanism may involve regulation of the binding of VIIa to TF through the lipid composition of the membrane surrounding TF. We have shown that TF localizes in lipid rafts, and it has been proposed that rafts are necessary for the tonic inhibition of TF-VIIa activity on the cell surface.45 In the current study, we have shown that TF-VIIa activity increases upon fusion of TF-bearing microvesicles and activated platelets. In such a scenario, transfer of TF from the microvesicle to the platelet, and its integration on the platelet membrane, may allow lateral diffusion of TF to nonraft membrane regions, where TF becomes decrypted and capable of initiating coagulation. In this way, the product of the TF-VIIa reaction, factor Xa, is formed in the very membrane where it will carry out the subsequent reaction in the coagulation cascade, converting prothrombin to thrombin. Alternatively, the transfer of TF to the platelet membrane may enable the cytoplasmic modification of TF to an active form by enzymes not present in the microvesicles. Either of these mechanisms (and possibly others) is consistent with our finding that the catalytic efficiency of the TF-VIIa complex increases when microvesicles fuse with activated platelets. Although the precise means by which TF is decrypted when the microvesicles fuse with platelets is as yet undetermined, the results nevertheless suggest a solution for limiting coagulation to sites of vessel injury where platelets become activated.
Prepublished online as Blood First Edition Paper, March 1, 2005; DOI 10.1182/blood-2004-03-1095.
Supported in part by grant 0 325 389Y from the American Heart Association (I.d.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Toon Stegmann (Toulouse, France) for help in designing the membrane fusion assay, Dr C. Wayne Smith (Baylor College of Medicine, Houston, TX) and Dr Gabriel Lopez-Berestein (MD Anderson Cancer Center, Houston, TX) for helpful discussions, and Dr Raul Tonda (current address, Hospital Clinic, Barcelona, Spain) for assistance with the figures.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal