Abstract
The field of vascular biology has been stimulated by the concept that circulating endothelial progenitor cells (EPCs) may play a role in neoangiogenesis (postnatal vasculogenesis). One problem for the field has been the difficulty in accurately defining an EPC. Likewise, circulating endothelial cells (CECs) are not well defined. The lack of a detailed understanding of the proliferative potential of EPCs and CECs has contributed to the controversy in identifying these cells and understanding their biology in vitro or in vivo. A novel paradigm using proliferative potential as one defining aspect of EPC biology suggests that a hierarchy of EPCs exists in human blood and blood vessels. The potential implications of this view in relation to current EPC definitions are discussed.
Introduction
During embryogenesis, blood vessels are formed de novo by the patterned assembly of angioblasts in a process termed vasculogenesis.1 Once an intact vascular system has been established, the development of new blood vessels occurs via the sprouting of endothelial cells from postcapillary venules or the maturation and de novo growth of collateral conduits from larger diameter arteries.2,3 These 2 mechanisms of new blood vessel formation are termed angiogenesis and arteriogenesis, respectively. In 1997, Asahara et al4 described a population of human circulating CD34+ cells that could differentiate ex vivo into cells with endothelial cell–like characteristics. These cells were termed “endothelial progenitor cells” (EPCs), and this landmark study challenged the traditional understanding of angiogenesis to suggest that circulating cells in adult peripheral blood may also contribute to new vessel formation. Further, subsequent studies showed that these cells are derived from bone marrow, circulate in peripheral blood, and home to sites of new blood vessel formation that include ischemic tissues and tumor microenvironments (reviewed in Urbich and Dimmeler,5 Rafii and Lyden,6 Khakoo and Finkel,7 Schatteman,8 Iwami et al,9 Hristov and Weber,10 and Murasawa and Asahara11 ). From these initial reports, intense effort has recently focused on defining the role of circulating marrow-derived EPCs in the repair of damaged vascular endothelium or in tumor angiogenesis and on translating these experimental observations into human clinical trials for repair of vascular injury and/or ischemic tissue or as a novel strategy for anticancer therapy.5-11
Using sophisticated cell-marking strategies, very recent studies indicate that marrow-derived EPCs may play minimal or no role in neovascularization of tumors, vessel repair, or normal vessel growth and development.12,13 These conflicting reports have raised questions about the function of EPCs in vascular homeostasis and repair. The controversies surrounding these fundamental questions may in part originate from the heterogenous phenotypic definitions of EPCs and a lack of functional clonogenic assays to isolate and accurately describe the proliferative potential of EPCs. A hallmark of many stem or progenitor cells in various tissues is their ability to give rise to numerous differentiated progeny to provide sufficient cells for tissue homeostasis. For example, a single hair follicle stem cell can generate as many as 1.7 × 1038 progeny in vitro,14,15 and a hematopoietic stem cell has been estimated to undergo up to a 720 000-fold expansion during the process of mature blood cell production.16 This kind of detailed analysis of the proliferative potential of EPCs has not been conducted in vitro or following transplantation in vivo and has remained a limitation in defining an EPC.5-7
There is another population of circulating cells, called circulating endothelial cells (CECs), that are of great interest as a biomarker for predicting the presence and severity of vascular disease.17 CECs are thought to represent mature nondividing endothelial cells of the vascular intima that are sloughed off following some form of metabolic, infectious, hemodynamic, or other pathologic process (reviewed in Blann18 ). While the roles that CECs may play in vascular pathology remain unclear, several reports indicate that enumerating the circulating number of CECs may be informative in understanding disease processes that result in potentially irreversible injury to the vessel wall.18-20
Because the use of EPCs for angiogenic therapies and the potential predictive value in assessing EPCs and CECs as biomarkers for cardiovascular disease risk and progression have been extensively reviewed elsewhere,5-11,17-20 this review focuses on 4 largely unresolved questions involving the biology of EPCs and CECs: (1) Can one quantitatively measure the proliferative potential of EPCs in vitro? (2) Do all EPCs possess the same proliferative potential? (3) Do endothelial cells present in the vascular intima possess proliferative potential or are mature vascular endothelial cells essentially nonmitotic? (4) Do CECs possess proliferative potential at a single-cell level?
Methods of isolating and quantitating EPCs and CECs
Interest in circulating angiogenic cells can be traced back at least 30 years to a series of papers describing circulating cells with the morphology of endothelial cells isolated in a variety of experimental models of vascular injury.21-24 Research progress in this area was hampered for many years by a lack of reagents to specifically identify the circulating cells as endothelial cells. While the discovery of monoclonal antibodies and the use of magnetic-bead immunoselection and/or fluorescence-activated cell sorting have improved our ability to isolate, enumerate, and characterize circulating cells, CECs and EPCs remain extremely rare in adult peripheral blood (0.01% and 0.0001%, respectively), and the paucity of these circulating cells has contributed to the lack of clearly defining methods for prospective cell isolation and definition.
The ability to culture endothelial cells or endothelial-like cells from peripheral blood was an important advance in the rekindling of interest in circulating angiogenic cells since these rare cells could now be isolated and expanded for further study. However, the peripheral blood contains several cell types that possess the ability to differentiate into cells with endothelial-like gene expression patterns in vitro, including hematopoietic stem cells (with hemangioblast potential), mononuclear phagocytes (monocyte-macrophages), and sloughed endothelial cells (ie, CECs) (reviewed in Schatteman and Awad25 ). The early methods used to culture the peripheral blood mononuclear cells to derive endothelial-like cells could not discriminate between these many sources of EPCs. For example, one common method for culturing EPCs from human peripheral blood is to plate the low-density mononuclear cells on fibronectin-coated plates using commercially available tissue-culture media with added endothelial growth supplements, vascular endothelial growth factor, and fetal calf serum.26 After 4 days of cultivation, the adherent cells are examined for expression of several characteristics of endothelial cells such as uptake of acetylated-low density lipoprotein (Ac-LDL) and binding of the plant lectin Ulex europaeus. These cells may also express the endothelial form of nitric oxide synthase, von Willebrand factor, vascular endothelial growth factor receptor-2 (kinase insert domain-containing receptor [KDR] or fetal liver kinase-1, and CD144.26 However, in most analyses, the adherent cells derived from peripheral blood mononuclear cells also express CD45 and in some cases CD11b, CD11c, CD14, and CD68, indicating a phenotype of a cell that is displaying molecules typical of macrophages and endothelial cells.27-29 Indeed, it is well known that monocytes will express a variety of “endothelial-like” proteins when cultured under specific conditions in vitro.30-35 In fact, macrophages are now being recognized as a particularly difficult cell type to define in end-stage forms, because these cells are extremely flexible in their phenotype and function and may best be described as continuously adapting their functional patterns in response to changing microenvironmental states.36 Thus, the choice of culture conditions are important, because culturing monocytes in the presence of certain myeloid growth factors known to promote distinct macrophage or dendritic cell phenotypes produces cells that do not promote neovascularization in vivo as significantly upon transplantation as do the mononuclear cells cultured in the “EPC” conditions.26 These results suggest that the EPCs derived from bone marrow cells may largely be derived from the mononuclear phagocyte lineage; one could even hypothesize that these cells represent a unique form of macrophage differentiation (angiogenic macrophage). Alternatively, the EPC may be derived from an earlier common myeloid progenitor and ultimately from the hematopoietic stem cell. In fact, such a relationship has been strongly supported in the murine system where transplantation of a single hematopoietic stem cell can repopulate the hematopoietic lineage and the endothelial lining of retinal blood vessels (in 4% of the animals that received transplants) following experimental retinal ischemia.37 A similar relationship between the human hematopoietic stem cell and the endothelial lineage has been conducted at a single-cell level in vitro.38 Because hematopoietic stem cells express a variety of angiogenic growth factor receptors39-42 and are known to play important angiogenic roles via the secretion of numerous growth factors and proteases at the site of new vessel formation, separating the specific roles of these cells from the EPCs in a heterogenous population may be problematic.
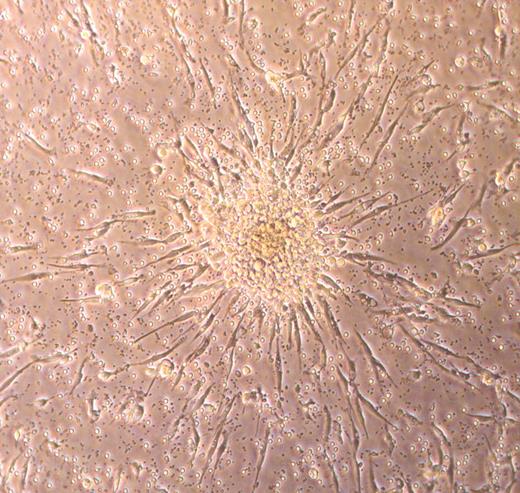
In an attempt to avoid possible contamination of EPC cultures with monocytes, hematopoietic progenitors, and/or CECs that may be in human peripheral blood, an alternative culture method uses a preplating step of 24 to 48 hours to remove all the adherent low-density mononuclear cells and then replates the nonadherent cells onto fibronectin-coated dishes.43 After 7 days of culture the plates are scanned for the presence of EPC colonies (Figure 1), and the number of colonies are counted. Colonies are defined as a cell mass composed of a central cord of round cells with elongated spindle-shaped cells sprouting at the periphery of the colony (Figure 1). These EPC-derived cells have been reported to express KDR, CD31, and Tie-2 (angiopoietin 1 receptor). It is unclear whether the colony-forming cells express CD45 or any of the other mononuclear phagocyte antigens. These EPC colonies also ingest Ac-LDL and bind plant lectins (a feature also displayed by most macrophages in culture in addition to the EPCs). This method of culture forms the fundamental basis for many of the EPC studies published to date.7 In fact, it is now possible to commercially obtain a cell-culture assay kit to identify these EPCs (referred to as endothelial cell colony-forming unit or CFU-EC) from peripheral blood mononuclear cell preparations.
One interesting feature of the colonies emerging from the EPCs is that these colonies disappear by day 10 to 14 in culture leaving behind the spindle-shaped adherent cells expressing the endothelial antigens.43 Subsequent studies have determined that the only way to permit peripheral blood mononuclear cell–derived colonies to continue to survive in vitro for up to 30 days is to transfect the adherent cells with the cDNA for human telomerase reverse transcriptase.44 While the spindle-shaped cells that emerge from the EPC colonies have been extensively phenotyped and studied, the proliferative potential of the cells has not been well examined. It appears that the total number of adherent cells in these cultures can expand somewhere between 8- and 90-fold during a period of 3 to 10 days.45-48 It is apparent that the nonadherent cells recovered after the preplating step cannot be passaged significantly ex vivo at a single-cell level because the cloning efficiency is only 3%.46 Our own unpublished studies (D.A.I. and M.C.Y., July 2005) corroborate the published work because we find that human mononuclear CD45+ adherent cells do not proliferate at a single-cell level and cannot be harvested as a population and passaged reproducibly. It remains unclear whether the low-cloning efficiency is related to suboptimal culture conditions or to the inherently low-proliferative potential of the isolated EPCs.
Photomicrograph of a typical endothelial cell colony-forming unit (CFU-EC) that is counted as an EPC.34 The cluster of round cells sits on top of spindle-shaped adherent cells that emigrate from the base of the cell cluster. Over time, the cluster of cells disappears, leaving the adherent spindle-shaped cells that display features of endothelial cells. Phase-contrast image was collected using a Zeiss Axiovert 2 inverted microscope (Carl Zeiss, Thornwood, NY) with a 5 × CP-ACHROMAT/0.12 NA objective. Image was captured with a SPOT RT color camera (Diagnostic, Sterling Heights, MI).
Photomicrograph of a typical endothelial cell colony-forming unit (CFU-EC) that is counted as an EPC.34 The cluster of round cells sits on top of spindle-shaped adherent cells that emigrate from the base of the cell cluster. Over time, the cluster of cells disappears, leaving the adherent spindle-shaped cells that display features of endothelial cells. Phase-contrast image was collected using a Zeiss Axiovert 2 inverted microscope (Carl Zeiss, Thornwood, NY) with a 5 × CP-ACHROMAT/0.12 NA objective. Image was captured with a SPOT RT color camera (Diagnostic, Sterling Heights, MI).
CECs will also appear in the adherent fraction of the cultured low-density mononuclear cells.49 These CD45– cells appear within a week of culture and also demonstrate low proliferative capacity as evidenced by an approximate 20-fold expansion in vitro. Lin et al49 used patients who received sex-mismatched bone marrow transplants to determine that the CECs appearing within a week of plating were most likely mature endothelial cells sloughed from the vessel wall, and they postulate that this may account for the limited growth capability of early-emerging CECs.
Another type of cell colony that can be isolated from mononuclear cells under similar cell culture conditions appears later in culture (between 14 and 21 days). Lin et al49 originally identified these cell colonies and called these CECs, late blood outgrowth endothelial cells, or endothelial outgrowth cells (EOCs) based on both their time of appearance in culture and their remarkable proliferative capacity. In contrast to the methods described for isolating EPCs (paragraph 2 of this section), Lin et al49 did not perform an adherence depletion step for macrophages and monocytes, but rather serially discarded the nonadherent cells. Therefore, the colonies that were isolated were derived from an adherent cell. EOCs displayed robust proliferative potential with 1000-fold expansion over 2 months ex vivo. Using patients who had received sex-mismatched bone marrow transplants, this study demonstrated that EOCs originate from human bone marrow and can be derived from long-term culture of mononuclear cells isolated from adult peripheral blood.
Since this original study, several groups have successfully isolated EOCs from both cord and adult peripheral blood.29,50-55 In contrast to other populations of EPCs or circulating angiogenic cells isolated in short-term culture of adult or cord blood mononuclear cells, EOCs uniformly express endothelial but not hematopoietic cell-specific surface antigens such as CD45 or CD14. Further, EOCs can be expanded for at least 30 population doublings in culture with serial passage, which are proliferative kinetics not associated with EPCs derived from nonadherent cells. The ability to serially passage EOCs may be a significant difference between these cells and all prior methods of isolating EPCs as early outgrowth EPCs (days 4-7) do not appear capable of being passaged serially. Thus, EOCs are not contaminated with hematopoietic cells and demonstrate extensive proliferative potential.
Hematopoietic progenitor cell paradigms and potential utility for classifying unique populations of EPCs
The assays outlined in the previous section identify several distinct populations of cells with angiogenic potential: (1) hematopoietic-derived EPCs, which coexpress hematopoietic and endothelial cell antigens and display limited proliferative potential; (2) CECs that express endothelial and no hematopoietic antigens but also display low proliferative potential; and (3) EOCs, which exclusively express endothelial cell–specific antigens and retain extensive proliferative potential. Until recently there has been no assay that permitted one to compare the proliferative potential of various EPC, CEC, or EOC populations and to determine whether each of these progenitors represents a heterogenous pool of cells with differing levels of proliferative potential or whether EPCs, CECs, and EOCs are distinct homogenous stages of endothelial cell development.
Hematopoietic cell progenitors have been defined by their ability to form colonies or small clusters of cells based on differences in proliferative and/or differentiation potential. Pluznik and Sachs56 and Bradley and Metcalf 57 initially reported that murine hematopoietic cells could be cultured in vitro and that addition of soluble fluid from different murine organs (ie, urine or pregnant uterine extract) resulted in the in vitro formation of myeloid colonies. Furthermore, these investigators reported that each myeloid colony developing in vitro arose from a single precursor cell called the colony-forming unit in culture or CFU-C. Use of this assay permitted identification of numerous hematopoietic growth factors and cytokines important for erythroid, myeloid, and multipotent progenitor cell proliferation and differentiation.
Plating of hematopoietic cells in special double-layer agar cultures with multiple recombinant cytokines also permitted the identification of clonal hematopoietic progenitors that were highly proliferative (high-proliferative potential–colony-forming cells or HPP-CFCs). In contrast to the more differentiated low-proliferative potential–colony-forming cell (LPP-CFC), HPP-CFC colonies contained greater than 50 000 cells and were visible in the culture dishes without need for magnification. HPP-CFC clones could be plucked from the agar medium, dispersed into a single-cell suspension, and replated in CFC assays with emergence of secondary HPP-CFCs, as well as committed erythroid and myeloid and multipotent progenitors.58 Because of these properties, HPP-CFCs are still considered the most primitive hematopoietic progenitor cell that can be cultured in vitro without the presence of hematopoietic stromal cells in coculture. It is important to point out that none of these colony assays identify the hematopoietic stem cell. A stem cell for the hematopoietic system is defined by its ability to repopulate the hematopoietic system of a recipient animal following adoptive transfer.59 Generally, such a transplantation requires that the host be myeloablated or that the host stem cells are genetically compromised in self-renewal or proliferative capacity for the donor cells to engraft and expand in the host animal.56,57
Thus, the hematopoietic system is organized as a hierarchy of cells that progress from the stem cell, possessing the most proliferative potential, through successive progenitor cell stages that sequentially lose mitotic potential but display increasing evidence of lineage commitment, to the final mature cells of each lineage that are highly differentiated and for the most part, devoid of proliferative potential. Having established this robust hierarchic order of progenitor cell activity (at a clonal level) using the in vitro assays, further studies of cell-surface antigen expression and in vivo functional analysis permitted prospective identification and further characterization of these hematopoietic progenitors (reviewed in Shizuru et al,59 Verfaillie,60 and Storb61 ).
Development of a novel clonogenic assay to quantitate the proliferative potential of single EPCs
While a hierarchy of hematopoietic stem and progenitor cells has been well established, evidence to support a similar hierarchy of progenitor cells (based on differences in proliferative potential) for the endothelial lineage has not been postulated. In a recent report, we hypothesized that a hierarchy of EPCs exists for the endothelial lineage.62 To address this question, we harvested mononuclear cells (MNCs) from peripheral blood of healthy adults and umbilical cords of healthy term infants and observed for endothelial colony formation using a modified protocol originally developed by the Hebbel laboratory for isolating EOCs.49 Of interest, the number of endothelial cell colonies per equivalent blood volume was increased 15-fold in cord blood compared with adult samples, and the cord blood–derived endothelial cell colonies emerged in culture 1 week earlier than adult colonies. These observations suggested that cord blood EPCs may be derived from a different population of progenitors than those previously identified as adult EOCs.
As a first estimate of the proliferative potential of the endothelial cell progeny derived from cord blood and adult endothelial cell colonies, cells were serially passaged in vitro. Remarkably, cord blood EPC-derived endothelial cells could be expanded for at least 100 population doublings without obvious signs of senescence. In contrast, adult EPC-derived endothelial cells could not be cultured far beyond 30 population doublings, which is consistent with previously published reports. As a second strategy to compare the growth potential of the EPC-derived endothelial cells, we replated cord blood and adult blood EPC-derived endothelial cells at 1 to 2 passages in limiting dilutions in culture medium defined for optimal endothelial cell growth in vitro. At cell concentrations of no more than 100/cm2, one could readily discern colonies of cells varying in size and cell morphology in addition to many single adherent endothelial cells. These data suggested that EPC-derived endothelial cells demonstrate a wide variability in proliferative and clonogenic potential and that some of the EPC-derived endothelial cells from cord blood display much higher proliferative potential than any of the endothelial cells derived from adult blood EPCs.
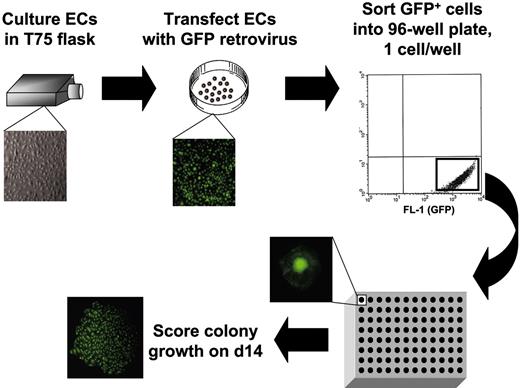
To quantitatively and stringently interrogate the proliferative potential of individual EPCs, we devised a single-cell assay for the clonal analysis of EPC colony-forming activity (Figure 2).62 Cord blood and adult blood EPC-derived endothelial cells at 1 to 2 passages were transduced with a retrovirus encoding enhanced green fluorescent protein (EGFP), and 24 hours later EGFP+ cells were removed from the plates and sorted as single cells into 96-well plates using a fluorescence activated cell sorting (FACS) Vantage sorter (100-μm nozzle and a sheath pressure < 9 pounds per square inch). After 2 weeks of culture, wells were examined to determine the percentage of wells that contained more than 1 cell and the total number of endothelial cells formed in each cell colony.
Remarkably, the percentage of single cells undergoing at least 1 cell division was increased 5-fold for cord blood EPC-derived endothelial cells compared with endothelial cells from adult EPCs. Further, the average number of cell progeny derived from cord blood EPC-derived endothelial cells individually plated was 100-fold greater compared with the number of cells derived from adult EPC-derived endothelial cells that were individually plated. Greater than 80% of the single adult endothelial cells that divided gave rise to small colonies or clusters of cells ranging in number from 2 to 50 cells. A small population of single adult endothelial cells did form colonies containing more than 500 cells. In contrast, at least 60% of the cord blood EPC-derived endothelial cells that were individually plated (that divided) formed well-circumscribed colonies containing between 2000 and 10 000 cells in the 14-day culture period. A representative of the photomicrograph of the colonies derived from single cord or adult cultured cells is shown in Figure 2. These single-cell studies demonstrated that there are different types of cord and adult EPC-derived endothelial cells, which could be discriminated by their cell autonomous proliferative potential, and that EPC-derived endothelial cells display a hierarchy of proliferative potentials similar to the hematopoietic progenitor cell hierarchy.
Method to identify the proliferative potential of individual endothelial cells (ECs). ECs derived from cord or adult blood EPCs were transduced with a retrovirus-encoding green fluorescence protein, the expressing cells were identified, and single cells were plated using a fluorescence-activated cell sorter equipped with a single-cell deposition device. The green fluorescing cells were visualized through a fluorescence microscope, and the number of cells in each well was directly counted. Microscope, objective, and camera details are as in Figure 1.
Method to identify the proliferative potential of individual endothelial cells (ECs). ECs derived from cord or adult blood EPCs were transduced with a retrovirus-encoding green fluorescence protein, the expressing cells were identified, and single cells were plated using a fluorescence-activated cell sorter equipped with a single-cell deposition device. The green fluorescing cells were visualized through a fluorescence microscope, and the number of cells in each well was directly counted. Microscope, objective, and camera details are as in Figure 1.
In the hematopoietic cell system, the most proliferative progenitor cell type that can be cultured in vitro in the absence of a stromal cell monolayer is termed the HPP-CFC. We tested whether a cell with similar proliferative potential is present within adult and cord blood EPC-derived endothelial cells to further define and classify the EPC hierarchy. The clonal progeny derived from a single plated cord blood or adult EPC-derived endothelial cell were trypsinized, replated, and cultured into 24-well tissue-culture plates for 7 days. After replating the clonal progeny of more than 1000 single adult EPC-derived cells (those primary wells containing > 50 cells), we detected only one secondary colony in the wells (1 of 1000 plated) after 14 days of culture. In contrast, approximately one half (205 of 421 plated) of the clonal progeny of single-plated cord blood EPC-derived endothelial cells (those primary wells > 50 endothelial cells) formed secondary colonies or rapidly grew to confluence in 24-well plates. Secondary colonies or confluent cell monolayers derived from single cord blood endothelial cells were serially passaged into progressively larger tissue culture plates. Strikingly, some single-plated cord blood EPC-derived endothelial cells yielded at least 1012 cells in long-term culture. These data suggested that the cord blood EPCs contained endothelial cells with additional EPC activity derived upon replating.
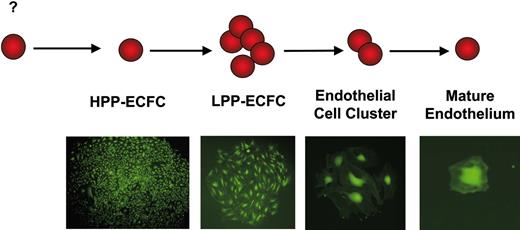
We have proposed that EPCs can be identified using similar terminology to that used for defining hematopoietic cell progenitors (Figure 3).62 HPP-ECFCs give rise to macroscopic colonies that form secondary and tertiary colonies upon replating. We have provided evidence that HPP-ECFCs give rise to all subsequent stages of endothelial progenitors in addition to replating into secondary HPP-ECFCs. LPP-ECFCs form colonies, which contain more than 50 cells, but do not form secondary LPP-ECFC colonies upon replating; this EPC stage represents the most proliferative population of EPCs that can be isolated from healthy adult peripheral blood. They do give rise to endothelial cell clusters. Endothelial cell clusters are composed of groups of fewer than 50 cells and do not replate into colonies or clusters. Our data have also indicated that mature differentiated endothelial cells can be identified in the single-cell assay and are defined as cells that are nonproliferative. Thus, the circulating EPCs emerging from cord and adult blood represent EPCs possessing different proliferative potentials. The fact that EPC-derived endothelial cells further displayed EPC activity raised the question of whether vessel-derived endothelial cells may also possess EPC activity.
Vessel-derived endothelial cells possess proliferative potential and represent EPCs that can be organized in a hierarchic fashion similar to circulating EPCs
Endothelial cell proliferation in normal, mature vessels in most mammals remains poorly defined, but in general it is reported to be extremely low. In fact, until approximately 50 years ago, the predominant view held that endothelial cells lining vessels do not undergo mitosis. However, the advent of tritiated thymidine–labeling studies and modifications of the hautchen preparation permitted direct analysis of endothelial cell mitosis in vessels recovered after labeling in vivo.63,64 In some experimental animals, such as rats, guinea pigs, pigs, and dogs, the tritiated thymidine–labeling studies demonstrated that 0.1% to 3.0% of endothelial cells proliferate daily.63-68 Endothelial cell proliferation rates were correlated with the age of the subject and appeared to decline rapidly after birth with most adult vessel endothelium displaying mitosis in less than 1% of the cells daily.69 Furthermore, the sites of endothelial cell replication were not homogenously distributed but appeared to occur in clustered areas nearest vessel bifurcations where flow was disturbed and often turbulent.64 Whether these dividing endothelial cells were unique and possessed proliferative potential that was lacking in other mature endothelium or these focal areas of replicating cells merely represented the sites of greatest vessel injury and endothelial turnover has not yet been determined. However, it has been well documented that endothelial cell division may reach 50% of the cells in and around the injured sites following experimentally induced hypertension, re-endothelialization of organized clots, or injured vessels after arterial denudation or following experimentally induced vascular constriction.68-70
Endopoiesis: the process of endothelial cell development. We propose that high-proliferative potential–endothelial colony-forming cells (HPP-ECFCs) are the most proliferative EPCs and that these cells demonstrate high replating potential. HPP-ECFCs can give rise to at least secondary HPP-ECFCs and all other EPCs and mature ECs. Low-proliferative potential–endothelial colony-forming cells (LPP-ECFCs) do not replate into secondary LPP-ECFCs but do form endothelial cell clusters and mature ECs. Endothelial cluster-forming cells give rise only to mature nondividing ECs. Mature ECs do not divide. Microscope, objective, and camera details are as in Figure 1. Cells were infected with a retrovirus encoding green fluorescence protein and thus appear green in the micrographs.
Endopoiesis: the process of endothelial cell development. We propose that high-proliferative potential–endothelial colony-forming cells (HPP-ECFCs) are the most proliferative EPCs and that these cells demonstrate high replating potential. HPP-ECFCs can give rise to at least secondary HPP-ECFCs and all other EPCs and mature ECs. Low-proliferative potential–endothelial colony-forming cells (LPP-ECFCs) do not replate into secondary LPP-ECFCs but do form endothelial cell clusters and mature ECs. Endothelial cluster-forming cells give rise only to mature nondividing ECs. Mature ECs do not divide. Microscope, objective, and camera details are as in Figure 1. Cells were infected with a retrovirus encoding green fluorescence protein and thus appear green in the micrographs.
In marked contrast to the slow turnover of endothelial cells in normal vessels, in vitro plating of endothelial cells derived from human or animal vessels is associated with rapid endothelial cell proliferation. Human umbilical vein endothelial cells (HUVECs) and human aortic endothelial cells (HAECs), which are both derived from blood vessels, are 2 commonly studied cell populations for in vitro analysis of endothelial cell functions. Although widely considered to be differentiated, mature endothelial cells, HUVECs and HAECs can be passaged for 40 to 60 population doublings in vitro, which are growth kinetics remarkably reminiscent of the endothelial progeny derived from adult and cord blood EPCs. On the basis of this paradox, we recently tested whether EPCs reside in HUVECs using the single-cell deposition assay.71
We compared the percentage of single-plated endothelial cells that divided at least once and the distribution of HPP-ECFCs, LPP-ECFCs, and endothelial clusters derived from cord blood EPC-derived endothelial cells, HUVECs, and HAECs. The percentage of EPC-derived endothelial cells individually plated undergoing at least one cell division was 55%. Similarly, 52% of single HUVECs and 53% of single HAECs divided after 14 days. Nearly half (47%) of the cord blood EPC-derived endothelial cells individually plated, which divided, possessed HPP-ECFC activity. Strikingly, 28% of single dividing HUVECs and 27% of single dividing HAECs, also possessed HPP-ECFC activity. In addition, some cord blood EPC-derived endothelial cells, HUVECs, and HAECs possessed LPP-ECFC activity and formed smaller colonies that contained between 51 and 500 cells. Thus, similar to the circulating EPCs, the endothelial cells lining umbilical veins and the human aorta are composed of resident EPCs at different stages of maturation possessing different levels of proliferative potential.
These data suggest a new conceptual framework for determining both the origin and function of EPCs in maintaining vessel integrity. Whereas HUVECs and HAECs have long been considered to be fully differentiated mature endothelial cells, these data now demonstrate that a complete hierarchy of EPCs can be identified in endothelial cells isolated from umbilical veins and adult aortas and discriminated by their clonogenic potential. Further, these data explain why HUVECs and HAECs can be passaged extensively in vitro given that HPP-ECFCs and LPP-ECFCs comprise nearly 50% of the HUVEC and HAEC populations. Future studies may clarify whether the sites of endothelial cell proliferation previously identified in the tritiated thymidine–labeling studies are directly linked to sites of EPC residence in the endothelium lining vessels in vivo.
Summation
In sum, it is apparent that the ability of EPCs to proliferate can be quantitatively analyzed at a single-cell level. Further, the use of a single-cell assay examining cellular proliferative potential indicates that endothelial cells derived from circulating progenitors or from the intimal layer of a vessel are organized in a hierarchic fashion similar to the hematopoietic system (and perhaps other adult stem cell systems). These cells do not appear to have any lineage relationship to the hematopoietic stem cells, but this remains to be formally proven.
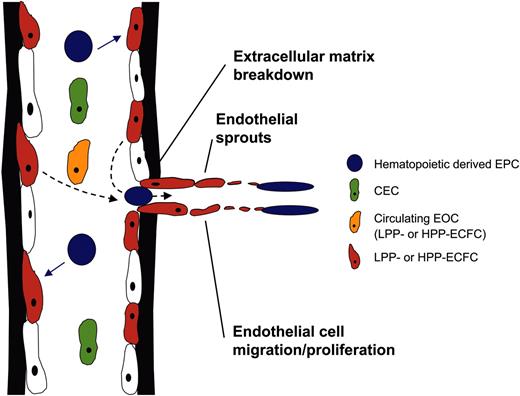
How do we incorporate this new information into the current definitions of EPCs, CECs, and EOCs? The term EPC, currently applied to those marrow-derived circulating cells that secrete angiogenic factors and participate in neoangiogenesis under certain stress (but probably not homeostatic) situations, appears to refer to cells that are derived from myeloid cells or earlier precursors of the hematopoietic lineage. The proliferative potential of these hematopoietic-derived EPCs will need to be examined in the single-cell assay, and their interaction with the HPP-ECFCs and other progenitors in the endothelial hierarchy must be further analyzed during new vessel formation. As predicted by several investigators,5-8,27-29,43,72,73 the hematopoietic-derived EPCs may serve as the earliest bloodborne angiogenic cells in the process of new vessel formation and may serve to stimulate the recruitment of host vessel–derived EPCs (Figure 4). CECs may represent single cells or clusters of endothelial cells that slough from the endothelial layer of vessels (during injury or stress), and some of these cells may harbor varying levels of proliferative potential (thus, CECs may not be uniformly mature nondividing endothelial cells). EOCs are likely to represent endothelial cells with medium-to-high proliferative potential that are present in circulating blood but appear to be marrow-derived according to Lin et al.49 Further studies will be required to determine the origin of the EOCs in the marrow (derived from the vascular endothelial intima of marrow vessels that may be carried along during a marrow harvest for transplantation or emerging from a precursor population in the extravascular marrow space). The vessel-derived EPCs (HPP-ECFCs, LPP-ECFCs, and endothelial clusters) are the most likely recruitable cells from formed vessels that contribute to new vessel formation (Figure 4) consistent with existing concepts of angiogenesis.
As occurred with development of the colony-forming assays for hematopoiesis, the single-cell endothelial colony-forming assay may serve as a fundamental tool to use in concert with monoclonal antibodies, FACSs, and/or immunomagnetic bead techniques, and cell transplantation strategies to further define each stage of endothelial progenitor cell biology prospectively during human development and in patients with diseases that affect vascular integrity. Will this assay identify the endothelial stem cell? Perhaps not, if the hematopoietic paradigm holds true (an in vivo repopulation assay will be required). However, this assay may also permit discovery of the key regulatory molecules that stimulate the EPCs to expand so vigorously ex vivo as compared with the strict quiescence state that these harvested EPCs are mired in as members of the vascular intimal layer in vivo. Such an analysis permits an understanding of not only the number of cells with proliferative potential in a vessel but also the distribution of the various states of proliferative progenitors—a factor that may prove informative as one of the primary variables underlying the progressive loss of angiogenic activity with aging.74-76
Model of the roles of EPCs, CECs, and EOCs in angiogenesis. Hematopoietically derived EPCs circulate and are the first cells to move into a site to facilitate the initiation and propagation of an angiogenic response. These angiogenic cells recruit LPP- or HPP-ECFCs that are residing in the endothelial intima in nearby vessels through the degraded vessel wall to form the endothelial sprouts. The roles played by the CECs and circulating EOCs are less clear, but these cells possess proliferative potential (may possess any level of progenitor activity from cluster to HPP-ECFCs, depending on the age of the host and whether the host is healthy or ill) and may well participate in neoangiogenesis.
Model of the roles of EPCs, CECs, and EOCs in angiogenesis. Hematopoietically derived EPCs circulate and are the first cells to move into a site to facilitate the initiation and propagation of an angiogenic response. These angiogenic cells recruit LPP- or HPP-ECFCs that are residing in the endothelial intima in nearby vessels through the degraded vessel wall to form the endothelial sprouts. The roles played by the CECs and circulating EOCs are less clear, but these cells possess proliferative potential (may possess any level of progenitor activity from cluster to HPP-ECFCs, depending on the age of the host and whether the host is healthy or ill) and may well participate in neoangiogenesis.
Prepublished online as Blood First Edition Paper, May 19, 2005; DOI 10.1182/blood-2005-04-1509.
Two of the authors (D.A.I. and M.C.Y.) have declared a financial interest in a company whose (potential) product was studied in the present work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal