In their paper, Miescher et al1 describe the effect of MonoRho, a recombinant anti-Rhesus D (RhD) human immunoglobulin G1 (IgG1)/kappa monoclonal antibody (mAb), on RhD+ red blood cell (RBC) elimination, and the association between this elimination and both FCGR2A and FCGR3A gene polymorphisms. They conclude that (1) there is a correlation between dose and mAb serum concentrations and an absence of correlation between mAb dose and RBC elimination, and (2) FCGR2A and FCGR3A genotypes influence this elimination.
A pharmacokinetic analysis using the measured mAb concentrations would have been useful to confirm the first assertion. However, Figure 1 of their paper clearly shows that concentrations do not increase linearly with the dose. Therefore, the analysis of the relationship between mAb dose and RBC elimination should have been based on mAb concentrations rather than dose, and on the calculation of RBC elimination rate over time rather than on estimation of global RBC half-life. In addition to studying the dose-response relationship, the authors should have taken into account individual genotypes as a covariable.
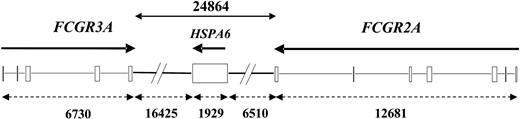
Relative positions of FCGR2A, HSPA6, and FCGR3A genes on chromosome 1 long arm. Boxes represent coding exons; thin lines, coding introns; and heavy lines, intergenic regions. Gene names are indicated above DNA with transcription direction (arrows). Genes and intergenic lengths (dashed arrows), based on where translation starts and ends, are indicated as base pairs below DNA, using AL5990385.22 GenBank sequence of RP11-5K23 BAC clone and identifying genes by nucleotides identity with GenBank cDNA sequences NM 021642, NM 002455, and NM 000569, respectively.
Relative positions of FCGR2A, HSPA6, and FCGR3A genes on chromosome 1 long arm. Boxes represent coding exons; thin lines, coding introns; and heavy lines, intergenic regions. Gene names are indicated above DNA with transcription direction (arrows). Genes and intergenic lengths (dashed arrows), based on where translation starts and ends, are indicated as base pairs below DNA, using AL5990385.22 GenBank sequence of RP11-5K23 BAC clone and identifying genes by nucleotides identity with GenBank cDNA sequences NM 021642, NM 002455, and NM 000569, respectively.
Miescher et al1 also report that FCGR3A-158V/F and FCGR2A- 131H/R genotypes are independently associated with RBC elimination. The influence of the FCGR3A polymorphism confirms our previously published results on the response of patients with non-Hodgkin lymphoma to rituximab, a chimeric IgG1 mAb.2 We showed that FCGR3A-158VV natural killer (NK) cells display a higher affinity for rituximab than FCGR3A-158FF, and that rituximab leads to higher antibody-dependent cellular cytotoxicity (ADCC) with FCGR3A-158VV than with FCGR3A-158FF NK cells.3 The influence of the FCGR3A genotype may be explained by a similar mechanism of action of these mAbs (ie, ADCC). The FCGR2A polymorphism is known to influence human IgG2, but not human IgG1, binding to the Fcγ receptor IIa (FcγRIIa).4 Therefore, the influence of the FCGR2A polymorphism is unexpected since the anti-RhD mAb under study was an IgG1. The reported influence of the FCGR2A genotype on RBC half-life was at the limit of significance (P = .05).1 In addition, the doses received by the subjects cannot be considered similar since their means were 975, 780, and 1440 μg for RR, HR, and HH patients, respectively (Kruskall-Wallis test, P = .098). The influence of the FCGR2A polymorphism observed by the authors can also be explained by the well-known linkage disequilibrium between FCGR3A-158 and FCGR2A-131 polymorphisms in white individuals.5 Indeed, both genes are physically very close together6 (about 25 kilobase pairs apart). We have confirmed this by analyzing the sequence of a bacterial artificial chromosome (BAC) clone available in public databases: the intergenic distance is only 24 863 base pairs (Figure 1). Despite the relatively small size of the population studied by Miescher et al,1 a nonrandom distribution of FCGR2A and FCGR3A genotypes can be detected when pooling FCGR2A-R carriers and FCGR3A-F carriers (Fisher exact test, P = .024).
Therefore, this study confirms the influence of the FCGR3A-158 polymorphism on the effect of cytolytic monoclonal antibodies but does not provide convincing results on an independent influence of the FCGR2A-131 polymorphism on the effect of this anti-RhD mAb.
Pharmacogenetics of monoclonal anti-Rhesus D antibody (MonoRho): effect of FCGR polymorphisms
In our study,1 we tested a monoclonal anti-Rhesus D (RhD) antibody (MonoRho) in RhD-negative volunteers challenged with RhD-positive erythrocytes. Elimination of RhD-positive erythrocytes was not dose dependent, but rather influenced by polymorphisms in the Fcγ receptor IIA (FCGR2A) and FCGR3A genes. Ternant and colleagues question the influence of the FCGR2A polymorphism and suggest additional evaluations, which we have now performed.
A possible effect of dose on the elimination of RhD-positive erythrocytes was investigated in an analysis of variance (ANOVA) with the following factors: dose, FCGR2A, FCGR3A, and FCGR3B. The dose effect was not statistically significant (P = .07), and there was no trend for increasing elimination speed with increasing dose levels. On the other hand, the P values for FCGR2A and FCGR3A became even more clearly significant (P = .02 for both versus P = .05 for both when dose was not entered in the model). The effect of FCGR3B remained nonsignificant (P = .64).
We repeated the ANOVA with the covariate serum concentration (area under the curve [AUC] from 1 to 48 h) and the factors FCGR2A, FCGR3A, and FCGR3B. This analysis showed no significant effect of the serum concentration (P = .13), while the effects of the FCGR2A and FCGR3A, but not of FGCR3B, were significant (P = .02, P = .04, and P = .97, respectively). The lack of a concentration effect is not unexpected, as the analytical method used measures only the antibody that has not bound to the target epitope. Additionally, saturation of anti-RhD binding sites was observed at all MonoRho doses except 300 μg.
Analyzing elimination rates rather than half-lives generates the same results, when both variables are log transformed.
The results of the ANOVA that we have performed rule out the possibility that the observed effect of the FCGR2A polymorphism was due to a linkage disequilibrium between FCGR2A and FCGR3A polymorphisms.
Thus, in our study the FCGR2A polymorphism had a statistically significant influence on the clearance of RhD-positive erythrocytes mediated by the monoclonal immunoglobulin G1 (IgG1) antibody MonoRho. However, there was no effect on the clinically more important end point of RhD sensitization. An effect of FCGR2A polymorphism on the clinical response to another IgG1 monoclonal antibody, rituximab, has been reported,2 while others have not seen such an effect.3-5
These observations are consistent with an independent effect of FCGR2A polymorphisms on antibody-mediated effector mechanisms. This effect may vary according to the monoclonal antibody, the effector mechanisms involved, the disease, and the end point of the study. Nevertheless, the possibility of a linkage disequilibrium with genes other than FCGR3A cannot be ruled out.
Further investigations are needed to clarify the effect of FcγR polymorphisms on the response to antibody therapies. In clinical trials, statistical tests such as analysis of variance or logistic regression should be used to test for effects of both polymorphisms, taking linkage disequilibrium into account. Clinical trials should be complemented with laboratory studies, which may help to unravel the different mechanisms and have important implications for the treatment of many diseases with monoclonal antibodies.
Correspondence: Sylvia Miescher, ZLB Behring AG, Wankdorfstrasse 10, CH-3000 Bern 22, Switzerland; e-mail: sylvia.miescher@zlbbehring.com; or Institute of Immunology, Inselspital, Bern CH 3010, Switzerland; e-mail: sylvia.miescher@dkf6.unibe.ch.
H.W. and G.P. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal