Abstract
Assessment of early therapeutic response using metabolic imaging is potentially useful to determine prognosis in aggressive lymphoma. Between January 2000 and January 2004, 90 patients with newly diagnosed aggressive lymphoma (median age 53 years, 94% diffuse large B-cell) were prospectively explored with [18F]fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) prior to induction chemotherapy, after 2 cycles (“early PET”), and after induction completion. Therapeutic response was evaluated using conventional diagnostic methods at 4 cycles. Induction treatment with an anthracycline-containing regimen was administered to all patients, associated with rituximab in 41%. According to the International Prognostic Index (IPI), 37 patients and 53 patients belonged to the lower- and higher-risk groups, respectively. At midinduction, “early PET” was considered negative in 54 patients and positive in 36. After completion of induction, 83% of PET-negative patients achieved complete remission compared with only 58% of PET-positive patients. Outcome differed significantly between PET-negative and PET-positive groups; the 2-year estimates of event-free survival reached 82% and 43%, respectively (P < .001), and the 2-year estimates of overall survival reached 90% and 61%, respectively (P = .006). Predictive value of “early PET” was observed in both the lower-risk and higher-risk groups, indicating prognostic independence from the IPI. Therefore, FDG-PET should be an early guide to first-line strategies in aggressive lymphoma. (Blood. 2005;106:1376-1381)
Introduction
Obtaining a complete remission (CR) after first-line chemotherapy is of paramount importance in patients with aggressive non-Hodgkin lymphoma because it usually leads to a longer progression-free survival, whereas an incomplete response is usually associated with a poorer outcome.1 Standardized criteria for response assessment have been proposed by Cheson and colleagues2 to ensure comparability among clinical trials, with these criteria relying mostly on measurement of tumor size using computed tomography (CT). However, in the presence of a residual mass, anatomic imaging is not optimal for discriminating active disease from fibrosis, and the positive predictive value of CT may be as low as 40%.3-5 This led to the concept of “complete remission uncertain” (CRu), which reflects the unknown significance of persistent radiologic abnormalities in patients who, otherwise, seem to be in CR.
By contrast, functional nuclear imaging provides metabolic tissue characterization, which is potentially more useful for response assessment after first-line chemotherapy. The emergence of [18F]fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) in the clinical armamentarium and its increasing availability have recently provided an alternative to [67Ga]gallium-citrate scan, which was previously used to detect residual active disease despite poor spatial resolution and low sensitivity at the abdominal level.6 With FDG-PET, several studies have demonstrated that persistence of an increased glycolytic activity in lymphoma lesions, that is, persistence of a positive scan at the end of first-line therapy, was associated with a 100% relapse rate, whereas the latter ranged from 16% to 20% in case of negative scan.3-5
Multiagent chemotherapy regimens have transformed aggressive lymphoma from a fatal disease to a potentially curable one, but no more than half of all patients are cured.7 More aggressive, but also potentially more toxic, treatments are now available; thus, there is an increasing interest for early patient selection, assuming that rapid responders to a standard induction are likely to show better and more durable response, whereas nonresponders could benefit from an early change of therapeutic orientation. The International Prognostic Index (IPI),8 a well-established predictor of outcome in aggressive lymphoma, is based on 5 pretherapeutic clinical characteristics: patient's age, Ann Arbor stage, serum lactate dehydrogenase level, performance status according to the Eastern Cooperative Oncology Group (ECOG) scale, and number of extranodal sites. However, recent gene profiling studies have shown that gene expression signatures can be used to predict the prognosis, independently from the IPI, a feature that may reflect the biologic heterogeneity observed in diffuse large B-cell lymphoma.9,10 In this respect, if FDG-PET could prove useful as an early indicator of tumor chemosensitivity, it might also reflect the heterogeneity of the disease better than the IPI and may help refine therapeutic strategies.
The purpose of the present study was to determine the early prognostic value of FDG-PET at midinduction in patients presenting with previously untreated aggressive lymphoma.
Patients, materials, and methods
Patient selection
Between January 2000 and January 2004, 93 patients with a newly diagnosed and histologically proven aggressive lymphoma were prospectively enrolled by 4 departments of hematology of the Assistance Publique-Hôpitaux de Paris (AP-HP), all being involved in the Groupe d'Etudes des Lymphomes de l'Adulte (GELA). Inclusion criteria were the following: adult younger than 80 years; diagnosis of diffuse large B-cell lymphoma or peripheral T-cell lymphoma as determined by a centralized histologic and phenotypic review11 ; measurable lesion; ECOG performance status of 0 to 2 and presence of at least one adverse prognostic factor of the age-adjusted IPI.8 Exclusion criteria were the following: meningeal involvement or primary cerebral lymphoma; positive serology for HIV; concomitant or previous cancer (except in situ cervical carcinoma); congestive heart failure or liver or kidney failure. According to the Declaration of Helsinski, the protocol was approved by our Institutional Review Board and all patients gave informed written consent. The study was sponsored by the Délégation à la Recherche Clinique de l'AP-HP.
Study design
Patients underwent FDG-PET, concurrently with CT of the chest, abdomen, and pelvis within a week, prior to induction treatment, after 2 cycles (“early PET”) and after the end of induction (4 cycles). Of importance, FDG-PET results did not influence the scheduled first-line therapeutic strategy. Response to induction was evaluated with conventional diagnostic methods (CDMs), consisting of clinical examination, CT, laboratory screening, and bone marrow biopsy if bone marrow was involved at baseline and classified according to the Cheson criteria.2 By May 2004, 90 patients with an “early PET” were eligible for analysis with a median follow-up of 2 years.
First-line therapeutic strategy
All patients received a doxorubicin-containing regimen as induction treatment, with or without rituximab, as detailed in Table 1. Patients older than 60 years were administered CHOP (cyclophosphamide, hydroxydaunomycin, Oncovin [vincristine], prednisone) or rituximab plus CHOP (R-CHOP) on the basis of 8 cycles every 3 weeks according to the LNH98-5 study of the GELA.13 Patients aged 60 years or younger and presenting with 1 to 3 adverse factors of the age-adjusted IPI received 4 cycles of CHOP-derived intensified regimens, namely, ACVBP (Adriamycin [doxorubicin], cyclophosphamide, vindesine, bleomycin, prednisone) or ACE (Adriamycin [doxorubicin], cyclophosphamide, etoposide) delivered every 2 weeks according to a GELA protocol currently active at that time.12 After closure of this protocol, subsequent patients received 4 cycles of a combination of rituximab and ACVBP (R-ACVBP) as an inductive regimen used in a recently activated GELA program. Finally, among the patients aged 60 years or younger presenting with 2 or 3 factors of the age-adjusted IPI, those who reached at least partial response on the basis of CDMs after induction (with ACVBP, ACE, or R-ACVBP),2 received a consolidative high-dose therapy followed by autologous peripheral blood stem cell transplantation (n = 36).
Initial patient characteristics and treatment according to PET status at 2 cycles
. | Total (n = 90) . | PET negative (n = 54) . | PET positive (n = 36) . |
|---|---|---|---|
| Median age, y (range) | 53 (17-78) | 52 (27-78) | 54 (19-76) |
| Sex, men/women | 56/34 | 37/17 | 19/17 |
| Histology, no. (%) | |||
| Diffuse large B-cell | 85 (94%) | 51 (94%) | 34 (94%) |
| Peripheral T-cell* | 5 (6%) | 3 (6%) | 2 (6%) |
| Performance status†, no. (%) | |||
| 0 | 34 (38%) | 25 (46%) | 9 (25%) |
| 1 | 27 (30%) | 14 (26%) | 13 (36%) |
| Greater than 1 | 29 (32%) | 15 (28%) | 14 (39%) |
| Ann Arbor stage, no. (%) | |||
| I-II | 8 (9%) | 6 (11%) | 2 (6%) |
| III-IV | 82 (91%) | 48 (89%) | 34 (94%) |
| Lactate dehydrogenase level more than 1 N, no. (%) | 58 (64%) | 31 (57%) | 27 (75%) |
| Extranodal localizations more than 1, no. (%) | 54 (60%) | 30 (56%) | 24 (67%) |
| Bulky disease more than 10 cm, no. (%) | 23 (26%) | 12 (22%) | 11 (31%) |
| Bone marrow involvement, no. (%) | 26 (29%) | 15 (28%) | 11 (31%) |
| Standard IPI score, no. (%) | |||
| Low risk (L) | 14 (16%) | 11 (20%) | 3 (8%) |
| Low-intermediate (LI) | 23 (26%) | 15 (28%) | 8 (22%) |
| High-intermediate (HI) | 30 (33%) | 17 (31%) | 13 (36%) |
| High (H) | 23 (26%) | 11 (20%) | 12 (33%) |
| Treatment regimen, no. (%) | |||
| CHOP | 3 (3%) | 1 (2%) | 2 (6%) |
| R-CHOP‡ | 24 (27%) | 16 (30%) | 8 (22%) |
| ACVBP/ACE12 | 50 (56%) | 27 (50%) | 23 (64%) |
| R-ACVBP | 13 (14%) | 10 (19%) | 3 (8%) |
. | Total (n = 90) . | PET negative (n = 54) . | PET positive (n = 36) . |
|---|---|---|---|
| Median age, y (range) | 53 (17-78) | 52 (27-78) | 54 (19-76) |
| Sex, men/women | 56/34 | 37/17 | 19/17 |
| Histology, no. (%) | |||
| Diffuse large B-cell | 85 (94%) | 51 (94%) | 34 (94%) |
| Peripheral T-cell* | 5 (6%) | 3 (6%) | 2 (6%) |
| Performance status†, no. (%) | |||
| 0 | 34 (38%) | 25 (46%) | 9 (25%) |
| 1 | 27 (30%) | 14 (26%) | 13 (36%) |
| Greater than 1 | 29 (32%) | 15 (28%) | 14 (39%) |
| Ann Arbor stage, no. (%) | |||
| I-II | 8 (9%) | 6 (11%) | 2 (6%) |
| III-IV | 82 (91%) | 48 (89%) | 34 (94%) |
| Lactate dehydrogenase level more than 1 N, no. (%) | 58 (64%) | 31 (57%) | 27 (75%) |
| Extranodal localizations more than 1, no. (%) | 54 (60%) | 30 (56%) | 24 (67%) |
| Bulky disease more than 10 cm, no. (%) | 23 (26%) | 12 (22%) | 11 (31%) |
| Bone marrow involvement, no. (%) | 26 (29%) | 15 (28%) | 11 (31%) |
| Standard IPI score, no. (%) | |||
| Low risk (L) | 14 (16%) | 11 (20%) | 3 (8%) |
| Low-intermediate (LI) | 23 (26%) | 15 (28%) | 8 (22%) |
| High-intermediate (HI) | 30 (33%) | 17 (31%) | 13 (36%) |
| High (H) | 23 (26%) | 11 (20%) | 12 (33%) |
| Treatment regimen, no. (%) | |||
| CHOP | 3 (3%) | 1 (2%) | 2 (6%) |
| R-CHOP‡ | 24 (27%) | 16 (30%) | 8 (22%) |
| ACVBP/ACE12 | 50 (56%) | 27 (50%) | 23 (64%) |
| R-ACVBP | 13 (14%) | 10 (19%) | 3 (8%) |
The chemotherapy regimens are as described in the text or as detailed in Haioun et al.12
Includes 1 case of angioimmunoblastic T-cell lymphoma and 4 cases of anaplastic large-cell lymphoma.
Eastern Cooperative Oncology Group (an increasing score indicates declining performance).
One patient with localized disease received 6 cycles instead of 8.
FDG-PET imaging
All patients underwent whole-body FDG-PET scanning prior to induction chemotherapy and after completion of the first 2 cycles (“early PET”), within the 48 hours preceding the third cycle, that is, with a median interval of 31 days after the first cycle and of 14 days after the second cycle. In addition, 80 patients (89%) underwent FDG-PET after completion of the 4 inductive cycles (“late PET”) with a median interval of 71 days after the first cycle and of 18 days after the fourth cycle. Patients fasted for at least 6 hours before each scan and were controlled by a blood glucose test value lower than 7 mM. They were given intravenous injections of 54 μCi/kg (2 MBq/kg) FDG and were asked to lie in the supine position for 1 hour to avoid muscular uptake. Imaging was performed on a dedicated C-PET scanner (ADAC, Milpitas, CA) featuring a 25-cm field-of-view and consisted of 5 to 7 overlapping bed shifts, to cover a volume starting from the upper thigh to the skull base. For each bed position, a 6-minute emission scan was acquired in a 3-dimensional coincidence mode, followed by a 1-minute transmission scan (137Cs source). Images were reconstructed iteratively with and without attenuation correction.
Image analysis
Images were interpreted by a consensus of 2 experienced observers blinded to clinical, radiologic, and follow-up data. All foci of abnormal FDG uptake were scored for their extent and intensity using a 3-point scale (1 = low, 2 = moderate, 3 = high) within each lymphatic area, organ, and skeletal region. Then, each postchemotherapy scan was scored as negative or positive. Negative was defined as having no residual abnormal uptake or as having a unique residual site (with an extent score of 1) associated with an intensity score of 1, whereas all the other previously hypermetabolic sites were extinguished. This approach has been successfully used by Mikhaeel et al in a previous study.4 Positive was defined as having at least one residual site (with an extent score of 1) associated with an intensity score of 2, or as having 2 or more residual sites with any extent and intensity scores.
Treatment evaluation and follow-up
Restaging was performed on the basis of CDMs after the first 2 and 4 inductive cycles, at the end of treatment, then every 6 months during 2 years, and yearly. The patient's status was assessed using standardized guidelines.2
Statistical analysis
The main objective of this study was to evaluate the role of FDG-PET performed at midinduction for predicting event-free survival and overall survival. Event-free survival was defined as the time interval from the date of enrollment in the study until progression, relapse, death, date of last follow-up, or stopping date (May 1, 2004). Overall survival was calculated from the date of enrollment until death from any cause. Data were censored at the date of the last evaluation when the stopping date was not reached. Univariate analysis was made using unpaired t test. Survival curves were estimated using the product-limit method of Kaplan-Meier and compared using the log-rank test. Differences were considered significant when the 2-sided P < .05. All statistical analyses were performed using the SAS software (version 8.0; SAS Institute, Cary, NC).
Results
Patient characteristics
Ninety patients (male-female ratio = 1.65) were eligible for analysis. Main clinical characteristics and allocated treatment regimens for the entire population and for the patient groups defined by the “early PET” status at 2 cycles (positive or negative) are summarized in Table 1. Histologically, 94% of patients had diffuse large B-cell lymphoma and, according to the IPI, 48% of patients presented with 3 or more adverse prognostic factors. Inductive chemotherapy consisted of an anthracycline-containing regimen, alone in 59% of patients or associated with rituximab in 41%.
Response to induction treatment
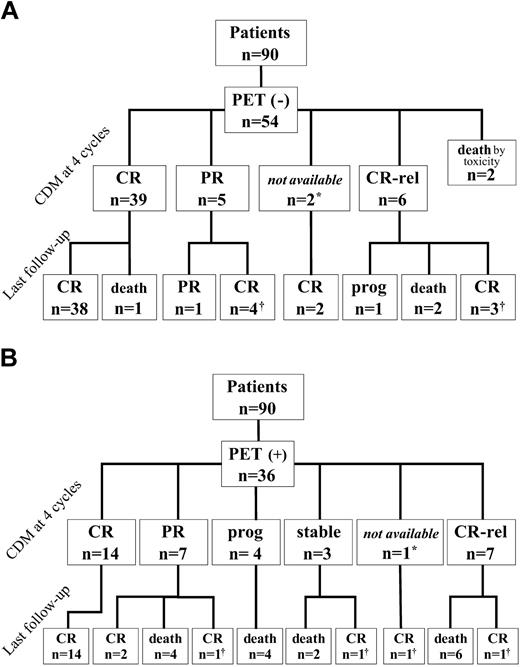
Pretherapeutic scans were strongly positive in all patients, showing at least one focus of uptake that scored 3 in intensity. After the first 2 cycles, 54 patients (60%) were considered “early PET” negative and 36 “early PET” positive (40%). Figure 1 shows, for the 2 groups, the results of the restaging at the end of induction treatment and at last follow-up, according to CDMs. After induction treatment (4 cycles), 45 of 54 “early PET”-negative patients (83%) were considered in CR or CRu on the basis of CDMs, of whom 38 remained in first CR at last follow-up. Conversely, only 21 of 36 “early PET”-positive patients (58%) achieved CR following induction treatment, of whom 14 remained in first CR at last follow-up.
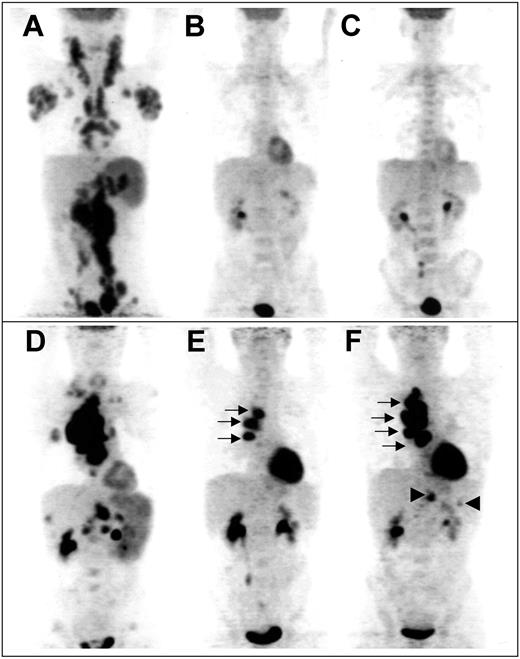
“Late PET” data were available in 80 patients. The reasons 10 patients were not explored at 4 cycles were 2 toxic deaths during the induction phase, one early withdrawal after 2 cycles due to aspergillosis, 3 disease progressions, and technical problems in 4 patients. Sixty patients were considered “late PET” negative after 4 cycles, among whom 52 were in CR on the basis of CDMs (case example in Figure 2A-C). Forty-seven of these 60 patients were previously “early PET” negative, whereas 13 patients who were initially “early PET” positive converted into “late PET” negative. The 2-year event-free survival and overall survival of these 13 patients were 85% and 83%, respectively. It is noteworthy that none of the “early PET”-negative patients became positive after 4 cycles. In addition, 20 of the 33 “early PET”-positive patients who were analyzed after 4 cycles remained positive (case example in Figure 2D-F); 9 of these patients were considered in CR on the basis of CDMs but 3 of them had relapses after 4, 11, and 12 months, respectively.
Clinical outcome of patients according to “early PET” status. Response to treatment is based on conventional diagnostic methods (CDMs) at 4 cycles and patient status is given at last follow-up. CR indicates complete remission; PR, partial remission; Rel, relapse; Prog, progression. *CT not performed; †CR obtained after salvage therapy.
Clinical outcome of patients according to “early PET” status. Response to treatment is based on conventional diagnostic methods (CDMs) at 4 cycles and patient status is given at last follow-up. CR indicates complete remission; PR, partial remission; Rel, relapse; Prog, progression. *CT not performed; †CR obtained after salvage therapy.
Outcome
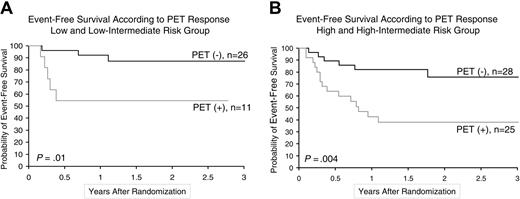
The 2-year event-free survival of the 54 “early PET”-negative patients was 82% (95% confidence interval [CI], 70%-93%), compared with only 43% (95% CI, 26%-59%) in the 36 “early PET”-positive patients (P < .001; Figure 3A). Overall survival also differed significantly (P = .006) between the 2 groups with 2-year estimates of 90% (95% CI, 81%-98%) and 61% (95% CI, 44%-79%), respectively (Figure 3B). Of importance, the prognostic impact of “early PET” on event-free survival was observed both among patients with low-risk disease (n = 37), indicated by an IPI score of 1 or 2 (P = .01), and those with high-risk disease (n = 53), indicated by an IPI score of 3, 4, or 5 (P = .004; Figure 4). The prognostic impact of “early PET” on event-free survival was also independent from the therapeutic regimen administered; it remained statistically significant (P < .001) in the subgroup of 63 patients who received intensified chemotherapy with or without rituximab (ACVBP/ACE, R-ACVBP), in the subgroup of 27 patients (P = .02) who received conventional chemotherapy with or without rituximab (CHOP, R-CHOP), as well as in the subgroup of 37 patients (P = .02) treated with rituximab (R-CHOP, R-ACVBP) and in the subgroup of 53 patients (P < .001) treated without rituximab (CHOP, ACVBP/ACE). Finally, “late PET” had an impact on event-free survival and overall survival with a magnitude similar to that of “early PET” (P < .001) given the fact that comparison was performed on 80 patients only.
Example of sequential FDG-PET findings in 2 patients. Panels A-C show scans from a patient with truly negative “early PET,” predicting CR. (A) Pretherapeutic scan shows diffuse involvement of jugular chains, axillae, mediastinum, mesenteric chains, spleen, and lateroaortic and iliac chains. No residual uptake is seen after 2 cycles (B) and after 4 cycles (C) of chemotherapy, whereas a medullar uptake is evidenced, due to hematopoietic activation. Panels D-F show scans from a patient with truly positive “early PET,” predicting relapse. (D) Pretherapeutic scan shows a cluster of hypermetabolic nodes in the right pulmonary hilum, supraclavicular and mesenteric nodes, and involvement of the spleen, right lung, liver, and lumbar spine. Three hilar foci persist after 2 cycles (E, arrows); their extent increases after 4 cycles (F, arrows), whereas subdiaphragmatic sites reappear (arrowheads), indicating progression of the disease.
Example of sequential FDG-PET findings in 2 patients. Panels A-C show scans from a patient with truly negative “early PET,” predicting CR. (A) Pretherapeutic scan shows diffuse involvement of jugular chains, axillae, mediastinum, mesenteric chains, spleen, and lateroaortic and iliac chains. No residual uptake is seen after 2 cycles (B) and after 4 cycles (C) of chemotherapy, whereas a medullar uptake is evidenced, due to hematopoietic activation. Panels D-F show scans from a patient with truly positive “early PET,” predicting relapse. (D) Pretherapeutic scan shows a cluster of hypermetabolic nodes in the right pulmonary hilum, supraclavicular and mesenteric nodes, and involvement of the spleen, right lung, liver, and lumbar spine. Three hilar foci persist after 2 cycles (E, arrows); their extent increases after 4 cycles (F, arrows), whereas subdiaphragmatic sites reappear (arrowheads), indicating progression of the disease.
Two-year estimates of survival according to “early PET” status. (A) Kaplan-Meier estimates of event-free survival. (B) Kaplan-Meier estimates of overall survival.
Two-year estimates of survival according to “early PET” status. (A) Kaplan-Meier estimates of event-free survival. (B) Kaplan-Meier estimates of overall survival.
Discussion
The present study demonstrates in a homogeneous series of patients with newly diagnosed aggressive lymphoma (diffuse large B-cell in the vast majority) that the assessment of metabolic activity by FDG-PET early after the onset of induction therapy can predict long-term prognosis. Indeed, patients with a negative scan are unlikely to have a relapse, whereas patients with a positive scan are likely not to be cured with the installed treatment. Thus, early identification of patients who have suboptimal chemotherapeutic responses might help to tailor treatment.
After a first publication by Janicek and colleagues14 in 1997, we have assisted in a growing interest for early restaging of lymphoma patients. The authors have shown, using [67Ga]gallium citrate in 30 patients with advanced stage aggressive lymphoma, that a positive scan half-way through chemotherapy (2 cycles of high-dose CHOP) had a much poorer outcome than those with a negative scan. Seventy percent of patients with negative interim scan remained disease free, whereas only 25% of gallium-positive patients at the same therapeutic point achieved durable remission. More recently, Israel and colleagues15 confirmed that gallium after 1 cycle was the most significant predictor of patient outcome, compared with pretreatment risk factors such as performance status, stage, and IPI, in 57 patients with aggressive lymphoma. Unfortunately, gallium scintigraphy is limited by its low sensitivity and by poor spatial resolution of γ cameras. In this setting, evaluation of residual disease, particularly at the abdominal level, is compromised by the physiologic tracer distribution demonstrating high uptake in the liver, spleen, and bowel. To overcome these limits, delayed images (later than 5 days after injection) are often required in the most difficult cases, which is senseless when rapid therapeutic decisions have to be made.6
Despite the important role of gallium, FDG has been shown to be a more effective agent. Several studies have established that an interim scan, performed after 1 to 4 cycles of chemotherapy, could provide predictive information in terms of response and survival.4,16-19 In a series of 11 patients, Römer and colleagues16 showed using standardized uptake value that tumoral FDG uptake was rapidly decreasing under conventional chemotherapy as early as 1 week after the onset of treatment. However, when measuring FDG uptake decrease at 6 weeks, correlation with clinical outcome was better than with the 1-week parameter. In a larger study of 28 patients, Jerusalem and colleagues17 have demonstrated that persistent tumoral FDG uptake after a median of 3 chemotherapy cycles was predictive of treatment failure, leading to shorten considerably the progression-free survival and overall survival. Unfortunately, the studied population was heterogeneous in terms of histologic subtypes, with one third of indolent follicular lymphoma and two thirds of aggressive diffuse lymphoma, as well as in terms of disease status, with one fifth of relapsed/transformed lymphoma and four fifths of previously untreated lymphoma. In a series of 49 patients with newly diagnosed aggressive lymphoma, of whom 23 underwent interim FDG-PET after 2 to 4 chemotherapy cycles, Mikhaeel and colleagues4 confirmed that the absence of residual FDG uptake (or minimal residual uptake) early during induction led to 0% of relapse, whereas a persistent tumoral uptake led to 87.5% relapse. Similar conclusions were later reached by Kostakoglu and colleagues18 in a 23-patient series where Hodgkin and non-Hodgkin lymphomas were mixed; an interim FDG-PET performed immediately after the first cycle of chemotherapy was predictive for the 18-month outcome. Of interest, when PET was performed at the end of treatment, the number of false-negative results increased. Finally, Spaepen and colleagues19 have explored the predictive value of persistent FDG uptake during treatment induction phase in a population of 70 patients with aggressive lymphomas, including only 47 diffuse large B-cell lymphomas. Thirty-six patients underwent FDG-PET after 3 cycles and 34 patients after 4 cycles. Thirty-three patients showed persistent abnormal FDG uptake and none of them achieved a durable CR, whereas 37 showed a negative scan, of whom 31 remained in CR at last follow-up. Comparison between groups indicated a statistically significant association between FDG-PET findings and progression-free survival and overall survival. Moreover, multivariate analysis indicated that FDG-PET at midtreatment was a stronger prognostic factor for progression-free survival and overall survival than the IPI.
Kaplan-Meier estimates of event-free survival according to “early PET” status and IPI. (A) Lower-risk group with IPI score 1 to 2. (B) Higher-risk group with IPI score 3 to 5.
Kaplan-Meier estimates of event-free survival according to “early PET” status and IPI. (A) Lower-risk group with IPI score 1 to 2. (B) Higher-risk group with IPI score 3 to 5.
The present study has some advantages over previous reports, in that it involves a larger and better targeted population (94% diffuse large B-cell lymphoma, age-adjusted IPI 1 or higher, CHOP-based treatments). The strong prognostic impact of a negative “early PET” is confirmed by an 82% event-free survival rate and 90% overall survival rate. Moreover, we have seen that the prognostic impact of “early PET” on event-free survival was true whether the therapeutic regimen was conventional or intensified, and whether it contained rituximab or not. However, the prognostic impact of a positive “early PET” is somehow contrasted compared with previous reports. Indeed, 14 patients with a positive FDG-PET at 2 cycles were finally found in CR at the end of induction therapy and remained in first CR throughout the follow-up. The main reason to explain this unexpected result could be the delay between the onset of treatment and PET imaging. Indeed, a trend for a shorter interval was found in this subset of patients, compared with the remainder of “early PET”-positive patients, 28.7 ± 4.9 days versus 31.6 ± 7.1 days (P = .19). Among these 14 patients, it is noteworthy that 9 converted into negative at the end of induction (after 4 cycles), which supports the hypothesis that an interval shorter than 10 days between the second cycle of therapy and PET imaging may generate false positive results. In a recent study, Spaepen and colleagues20 have shown that the stromal reaction following a chemotherapy cycle is maximal between days 3 and 10 and that this reaction is responsible for FDG uptake by macrophages, mimicking persistent viable tumor in sites of lymphoma.
Our results, obtained in an homogeneous population of aggressive lymphomas, confirm that the use of FDG-PET early after the onset of induction therapy can add further predictive power to the IPI (Figure 4). These findings support the concept of early stratification through FDG-PET to propose a more risk-adapted approach of treatment of aggressive lymphoma. Indeed, early identification of high-risk patients through the combination of FDG-PET and existing pretherapeutic prognostic parameters, such as immunohistochemical criteria,21-24 and—in the future—molecular signatures,9,10 could lead to early implementation of intensive (reinforced) therapies and improved clinical outcomes.
Prepublished online as Blood First Edition Paper, April 28, 2005; DOI 10.1182/blood-2005-01-0272.
Supported by a grant from the Programme Hospitalier de Recherche Clinique (PHRC-AOM00152), Ministry of Health, France.
C.H. and E.I. contributed equally to this study.
Presented in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 6-9, 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to the entire team of the AP-HP PET center for its valuable help with PET imaging, especially Prof J. N. Talbot, director, and Drs F. Montravers, D. Grahek, and K. Kerrou, Hôpital Tenon, Paris, France. We address particular thanks to the following collaborators who participated in the management of patients: Drs M. Diviné, T. El Gnaoui, I. Gaillard, B. Joly, J. Dupuis, C. Malhaire, M. Bouanane, B. Zegai, and A. Luciani, Hôpital Henri Mondor, Créteil; Dr J. Brière, Prof C. Gisselbrecht, Dr N. Mounier, Hôpital Saint-Louis, Paris; Drs M. Aoudjane and B. Fabiani, Hôpital Saint-Antoine, Paris; Dr J. Gabarre and F. Charlotte, Hôpital Pitié-Salpêtrière, Paris. We gratefully thank M. H. Valers for patient scheduling, E. Lebreton and A. Allain for their help with data management, Karine Sardin for her help in pathologic studies, and N. Nio for his help with statistical analyses.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal