Abstract
Current dendritic cell (DC)–based vaccines are based on ex vivo–generated autologous DCs loaded with antigen prior to readministration into patients. A more direct and less laborious strategy is to target antigens to DCs in vivo via specific surface receptors. Therefore, we developed a humanized antibody, hD1V1G2/G4 (hD1), directed against the C-type lectin DC-specific intercellular adhesion molecule 3–grabbing nonintegrin (DC-SIGN) to explore its capacity to serve as a target receptor for vaccination purposes. hD1 was cross-linked to a model antigen, keyhole limpet hemocyanin (KLH). We observed that the chimeric antibody-protein complex (hD1-KLH) bound specifically to DC-SIGN and was rapidly internalized and translocated to the lysosomal compartment. To determine the targeting efficiency of hD1-KLH, monocyte-derived DCs and peripheral blood lymphocytes (PBLs) were obtained from patients who had previously been vaccinated with KLH-pulsed DCs. Autologous DCs pulsed with hD1-KLH induced proliferation of patient PBLs at a 100-fold lower concentration than KLH-pulsed DCs. In addition, hD1-KLH–targeted DCs induced proliferation of naive T cells recognizing KLH epitopes in the context of major histocompatibility complex (MHC) classes I and II. We conclude that antibody-mediated targeting of antigen to DCs via DC-SIGN effectively induces antigen-specific naive as well as recall T-cell responses. This identifies DC-SIGN as a promising target molecule for DC-based vaccination strategies.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that play a key role in regulating antigen-specific immunity. DCs capture antigens, process them into peptides, and present these to T cells.1 The interaction between DC and T-cell controls the type and magnitude of the resulting immune response. Recently, preclinical and clinical studies have exploited DCs in an attempt to improve vaccine efficacy.2 Most of these studies involve ex vivo antigen loading of autologous monocyte-derived DCs that are readministrated to the patient, a laborious and costly procedure. A more direct strategy involves targeting of antigens specifically to antigen uptake receptors on the DC in vivo. Potential candidate receptors highly expressed by DCs include Fc receptors3-5 and members of the C-type lectin family.6,7 Whereas Fc receptors are expressed by many different cell types, the expression of some members of the C-type lectin family are more DC restricted.8
C-type lectins bind sugar residues in a calcium-dependent manner via a highly conserved carbohydrate recognition domain. C-type lectin receptors expressed by DCs are implicated in immunoregulatory processes, such as antigen capture, DC trafficking, and DC–T-cell interactions.8 Based on the location of the amino (N) terminus, 2 types of membrane-bound C-type lectins can be distinguished on DCs. Type I C-type lectins have their N terminus located outside, while type II C-type lectins have their N terminus located inside the cell. Several studies have been conducted on antigen targeting to C-type lectin receptors for vaccination purposes, mainly focusing on the type I C-type lectins mannose receptor (MR)9 and DEC-205.6,10,11 Vaccines based on natural MR ligands have been shown to effectively induce humoral and cellular responses.9 However, these ligands lack specificity for the MR, and may target multiple lectins with overlapping binding specificities, including soluble lectins and lectin receptors expressed by cells that are not specialized in antigen presentation. More specific receptor targeting can be obtained by the use of antibodies directed against specific C-type lectins, a strategy that has been successfully applied in MR7,12 and DEC-2056,10,11 targeting studies. Antibody-mediated targeting of antigen to the MR on human DCs results in antigen presentation and activation of naive T cells in vitro.7,12 Moreover, in vivo studies on antibody-mediated targeting of DEC-205 in mice demonstrate presentation of the antigen to naive CD4+ and CD8+ T cells.6,10
DC-specific intercellular adhesion molecule 3 (ICAM-3)– grabbing nonintegrin (DC-SIGN) represents a member of the type II C-type lectin family. We have previously demonstrated that DC-SIGN is an endocytic receptor mediating antigen presentation.13 A major advantage of targeting DC-SIGN over other C-type lectin receptors is its expression pattern. In humans, DC-SIGN expression is restricted to professional APCs and expression levels are high. Human DC-SIGN is abundantly expressed by DCs residing in lymphoid tissues and at mucosal surfaces, dermal DCs, and by specialized macrophages in placenta and lung.14,15
Targeting constructs that are to be used in humans should consist of antibodies that do not elicit immune responses directed against the antibody itself. Recent developments in antibody engineering provide the tools for the production of either humanized or human antibodies.16 Undesired interactions between the targeting antibody and Fc receptors can be avoided by use of single-chain Fv constructs or composite immunoglobulin G (IgG) molecules,17 thus enhancing targeting specificity.
Here we evaluate the effectiveness of targeting antigen to human DCs via DC-SIGN. For targeting purposes, keyhole limpet hemocyanin (KLH), a large globular protein containing a large array of immunogenic epitopes, was chosen as a model antigen. KLH is widely used in clinical DC-based vaccination trials for immunomonitoring purposes, and is thought to stimulate cytotoxic T-cell responses by recruiting bystander T-cell help.2 KLH was chemically cross-linked to a humanized anti–DC-SIGN IgG2/IgG4 composite antibody (hD1), resulting in the chimeric hD1-KLH protein. The results demonstrate that hD1-KLH was capable of inducing T-cell responses at a 100-fold lower concentration than KLH alone.
Patients, materials, and methods
Antibodies and reagents
The following antibodies were used: AZN-D1 (IgG1, mouse antihuman DC-SIGN),14 AZN-L19 (IgG1, mouse antihuman CD18),18 W6/32 (IgG2a, mouse anti–HLA-A, –HLA-B, –HLA-C; ATCC, Manassas, VA), IVA-12 (IgG1, mouse anti–HLA-DR, –HLA-DP, –HLA-DQ; ATCC), mouse IgG1 isotype (R&D Systems, Abingdon, United Kingdom), total mouse IgG (Jackson ImmunoResearch; Brunschwig Chemie B.V., Amsterdam, the Netherlands), Alexa Fluor 647–labeled goat anti–human IgG (Molecular Probes, Leiden, the Netherlands), and Alexa Fluor 647–labeled goat anti–mouse IgG1 (Molecular Probes). Endotoxin-free KLH was purchased from Calbiochem (La Jolla, CA).
Recombinant antibodies
The humanized antihuman DC-SIGN antibody hD1V1G2/G4 (hD1) was generated by complementarity determining region (CDR) grafting of AZN-D1 hypervariable domains into human framework regions. The humanized variable heavy and variable light regions were then genetically fused with a human hybrid IgG2/IgG4 constant domain17 and a human kappa chain constant domain, respectively. This construct was cloned into a mammalian expression vector and the final construct transfected into NSO cells. Stable transfectants were obtained using glutamate synthetase (GS) selection (Lonza Biologics, Portsmouth, NH). Supernatants containing hD1 were purified over a protein A column. An isotype control antibody, h5G1.1-mAb (5G1.1, eculizamab; Alexion Pharmaceuticals) containing the same IgG2/IgG4 constant region, is specific for the human terminal complement protein C5.19
Generation of hD1-KLH
The chemical cross-linker sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (sSMCC; Pierce, Rockford, IL) was conjugated to KLH according to the manufacturer's protocol. Protected sulfhydryl groups were introduced to the hD1 antibody with N-succinimidyl-S-acetylthiopropionate (SATP; Pierce) and were reduced with hydroxylamine hydrochloride (Pierce) using the manufacturer's protocol. Subsequently, hD1 was added to sSMCC-treated KLH in phosphate-buffered saline (PBS, pH 7.4) and allowed to react for 16 hours at 4°C. Unbound sites were alkylated by adding iodoacetamide (Sigma-Aldrich, St Louis, MO) to a final concentration of 25 mM, followed by 30-minute incubation at room temperature. The protein mixture was loaded onto a Superose 6 column (24-mL bed volume; Amersham Pharmacia Biotech, Uppsala, Sweden), and fractions were collected and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Fractions containing hD1-KLH were pooled and fractions containing free hD1 were discarded. The efficiency of the cross-linking reaction was estimated by comparing the amount of hD1 relative to KLH before the reaction to the hD1–to–hD1-KLH ratio after cross-linking. We calculated that, on average, each KLH molecule had reacted with 10 hD1 molecules (data not shown). Endotoxin levels of the pooled hD1-KLH fractions were below detection levels (< 0.04 pg/μg protein) in the QCL-1000 Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD).
Monocyte-derived DCs and PBLs
Peripheral blood mononuclear cells (PBMCs) were obtained from buffy coats of healthy individuals and were purified using Ficoll density centrifugation. Peripheral blood lymphocytes (PBLs) and immature DCs (iDCs) were obtained from PBMCs as reported elsewhere.14 In brief, PBMCs were allowed to adhere for one hour at 37°C. Nonadherent cells (PBLs) were gently removed, washed, and cryopreserved. The adherent monocytes were cultured in the presence of interleukin-4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF, 500 and 800 U/mL, respectively; Schering-Plough International, Kenilworth, NJ) for 6 days to obtain immature DCs. Mature DCs (mDCs) were obtained by culturing iDCs in the presence of 2 μg/mL lipopolysaccharide (LPS) for 24 hours. DCs were cryopreserved until use. Unless indicated otherwise, cells were cultured in X-VIVO 15 medium (Cambrex, Verviers, Belgium) supplemented with 2% human serum.
Tritiated thymidine incorporation assays
Tritiated thymidine (1 μCi [0.037 MBq]/well; MP Biomedicals, Amsterdam, the Netherlands) was added to the cell cultures. Tritiated thymidine incorporation was measured after 16 hours in a β-scintillation counter. Proliferation indices higher than 2 were considered positive.
Clinical vaccination protocol
PBLs and monocyte-derived DCs were isolated from melanoma patients participating in a clinical vaccination trial, as described by de Vries et al.20 Patients were determined to have stage IV disease according to the American Joint Committee on Cancer (AJCC) criteria.21 The study was approved by the institutional review board (Radboud University Nijmegen Medical Centre, Commissie Mensgebonden Onderzoek). Informed consent was provided according to the Declaration of Helsinki. The vaccination protocol consisted of 2 parts. In the first part, antigen-pulsed mDCs were administered intravenously and intradermally, 3 times at biweekly intervals. In the second part, patients received 3 monthly intradermal vaccinations with peptides alone (100 μg) and KLH (2 μg). Patients who remained free of disease progression after the first vaccination cycle were eligible for maintenance cycles at 6-month intervals, each consisting of 3 biweekly intranodal vaccinations in a clinically tumor-free lymph node region under ultrasound guidance with mDCs alternately pulsed with wild-type or modified glycoprotein 100 (gp100) peptides,20 tyrosinase peptides, and KLH. Clinical grade DCs for vaccination purposes were generated from PBMCs as described previously.22
Humoral responses to KLH
Humoral responses to KLH were determined by enzyme-linked immunosorbent assays (ELISA) as described by Holtl et al.23 Briefly, 96-well plates were coated overnight at 4°C with the protein KLH (25 μg/mL) in PBS (0.1 mL/well). Subsequently, plates were incubated with serial dilutions of patient serum, obtained before and during the third maintenance cycle, for one hour at room temperature. After extensive washing, human IgG-specific antibody labeled with horseradish peroxidase was allowed to bind for one hour at room temperature. Peroxidase activity was revealed using 3,3′ 5,5′ tetramethyl-benzidine as substrate and measured in a microtiter plate reader at 450 nm. A signal detected at 1:400 or higher dilution of serum was considered positive.
Cellular responses to KLH
Cryopreserved PBMCs, isolated from peripheral blood samples taken from the patients, were thawed, washed, and plated at 1 × 105 PBMCs per well of a 96-well tissue culture microplate either in the presence or absence of 10 μg/mL KLH. After 4 days of culture, a tritiated thymidine incorporation assay was performed.
Binding and internalization assays
Binding of hD1 and hD1-KLH to iDCs was assessed by immunofluorescence and flow cytometry. iDCs were incubated with or without 10 μg/mL hD1 or hD1-KLH. In some experiments, DC-SIGN was blocked by pretreating iDCs with 100 μg/mL AZN-D1. After a one-hour incubation at 4°C, cells were washed and incubated with Alexa Fluor 647–labeled anti–human IgG antibody. Cells were analyzed on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences, San Jose, CA).
Internalization of hD1 by iDCs was determined by flow cytometry as described previously.24 Briefly, iDCs were incubated with 10 μg/mL hD1, AZN-D1, AZN-L19, or mouse IgG1 and 5G1.1 isotype control antibodies at 4°C for 30 minutes, washed, and incubated for 0, 15, 30, or 45 minutes at 37°C. Subsequently, some of the cells were fixed, while others were fixed and permeabilized in PBS/0.1% (vol/wt) saponin (Sigma-Aldrich) before addition of the Alexa Fluor 647–labeled anti–human IgG secondary antibody. The amount of internalized antibody was calculated by subtracting the mean fluorescence in fixed cells (surface bound) from that recorded with fixed and permeabilized cells (internalized and surface bound) at the various time points.
Internalization of hD1 and hD1-KLH was confirmed by confocal laser scanning microscopy (CLSM). iDCs were incubated with 10 μg/mL hD1, hD1-KLH, AZN-D1, or isotype control 5G1.1 and mouse IgG1 antibodies for one hour at 37°C. Cells were fixed on poly-l-lysine–coated glass slides, followed by intracellular staining with Alexa Fluor 647–labeled secondary antibodies. Cells were imaged with a Bio-Rad MRC 1024 confocal system operating on a Nikon Optiphot microscope and a Nikon × 60 planApo 1.4 oil immersion lens (Bio-Rad, Hercules, CA). Pictures were analyzed with Bio-Rad Lasersharp 2000 and Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA) software.
KLH binding and internalization by iDCs was assessed by direct labeling of KLH using the Alexa Fluor 488 labeling kit (Molecular Probes). iDCs were incubated with 10 μg/mL labeled KLH for one hour at either 4°C or 37°C. Subsequently, cells were washed and analyzed by flow cytometry.
Live imaging of hD1-KLH and KLH uptake by iDCs
iDCs were labeled with LysoTracker Red (Molecular Probes) in PBS for 10 minutes at room temperature. Subsequently, cells were transferred to RPMI 1640 without phenol red (Gibco, Life Technologies, Breda, the Netherlands) supplemented with 1% human serum. Labeled cells were analyzed at 37°C with a Zeiss LSM 510 microscope equipped with a type S heated stage CO2 controller and PlanApochromatic 63 × 1.4 oil immersion DIC lens (Carl Zeiss, Jena, Germany). KLH and hD1-KLH, directly labeled with the Alexa Fluor 488 labeling kit (Molecular Probes), were added to the medium at 10 μg/mL. Cells were imaged using Zeiss LSM Image Browser version 3.2 (Carl Zeiss) and processed with Image J version 1.32j software (National Institutes of Health, http://rsb.info.nih.gov/ij).
Targeting experiments
iDCs were incubated with hD1, hD1-KLH, or KLH for one hour at 4°C or 37°C. Where indicated, iDCs were matured with LPS. Subsequently, DCs were washed and cocultured with KLH-responsive PBLs (ratio 1:10) at 37°C. In some experiments, iDCs were matured with LPS before addition of PBLs. After 16 hours of coculture, IL-2, IL-4, IL-5, IL-10, tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ) were measured by cytometric bead array (CBA) (T helper 1 [Th1]/Th2 Cytokine CBA 1; BD Pharmingen, San Diego, CA) according to the manufacturer's instructions. After 4 days of coculture, a tritiated thymidine incorporation assay was performed.
Presentation of KLH epitopes over time
iDCs were incubated with 5 μg/mL KLH or 5 μg/mL hD1-KLH for one hour at 37°C. Following washing, KLH-responsive PBLs were added to the iDCs either on the same day, or 2 or 4 days later. Four days after addition of the PBLs, proliferative responses were determined in a tritiated thymidine incorporation assay.
Activation and expansion of naive T cells
Experiments were performed essentially as described previously.7 Briefly, PBLs from a healthy donor were used as a source of T cells. Autologous DCs were incubated with 10 μg/mL hD1-KLH for one hour at 37°C, washed, and matured. Targeted mDCs were incubated with PBLs (ratio, 1:10) in the presence of IL-7 (10 ng/mL, day 0), followed by addition of IL-10 (10 ng/mL) on day 1 and IL-2 (20 U/mL) on day 2. IL-2 was added to the culture every 3 to 4 days. PBLs were restimulated each week. Restimulations were performed with iDCs treated with 5 μg/mL hD1-KLH after 1 week, and with iDCs treated with 2.5 μg/mL hD1-KLH after 2, 3, and 4 weeks. Finally, PBLs were harvested and cocultured with autologous mDCs that had been pulsed with KLH, as described in “Targeting experiments.” Proliferative responses were determined by tritiated thymidine incorporation assays.
Results
Humanized anti–DC-SIGN antibody hD1 is internalized by iDCs
The CDRs of the mouse antihuman DC-SIGN antibody AZN-D1 were grafted onto a human IgG2/IgG4 composite antibody to generate a humanized antibody for DC-SIGN targeting, hD1. We have previously shown that the human hybrid IgG2/IgG4 constant domain prevents antibodies from binding to Fc receptors.17 The binding affinity of hD1 for DC-SIGN was similar to that of AZN-D1 as determined by surface plasmon resonance (3.7 ± 0.7 nM and 3.8 ± 1.1 nM, respectively, data not shown).
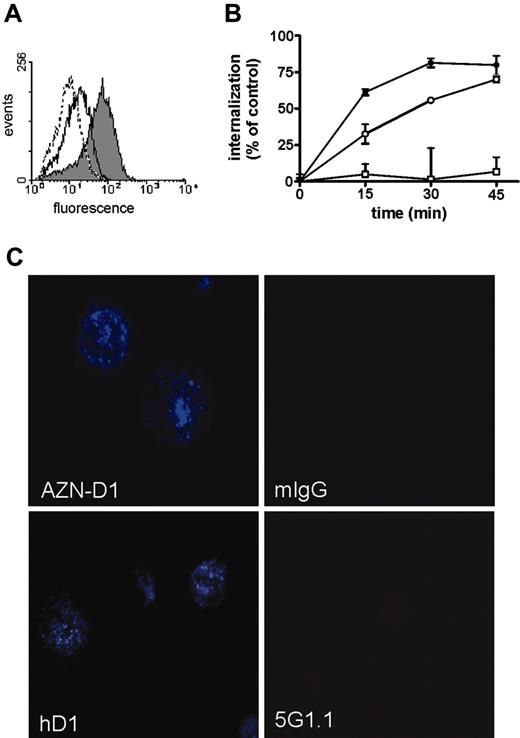
Flow cytometric analysis revealed specific binding of hD1 to DC-SIGN on iDCs, as preincubation of iDCs with AZN-D1 efficiently reduced binding (Figure 1A). Furthermore, hD1 bound to DC-SIGN–expressing K562 cells after transfection with DC-SIGN cDNA, whereas it did not bind to untransfected K562 cells (data not shown). A time-course internalization experiment revealed that both the hD1 and the AZN-D1 antibodies were rapidly internalized by iDCs, although the AZN-D1 antibody was internalized slightly more efficiently than hD1. As expected, the control AZN-L19 antibody, directed against CD18, was not internalized (Figure 1B). Analysis by confocal microscopy confirmed internalization of the hD1 and AZN-D1 antibodies by iDCs, whereas the control antibodies 5G1.1, directed against human terminal complement protein C5, and mouse IgG1 isotype were not internalized (Figure 1C).
hD1-KLH is rapidly internalized and translocated to the lysosmal compartment
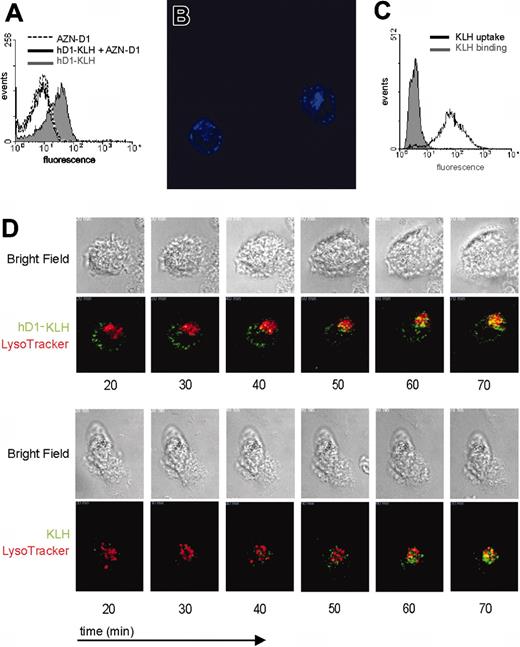
To further explore the potential of DC-SIGN as a targeting receptor for DC vaccination, we studied the uptake of KLH complexed to the humanized anti–DC-SIGN antibody hD1. As was observed with the hD1 antibody, hD1-KLH bound to iDCs (Figure 2A). hD1-KLH binding to DCs is mediated by DC-SIGN, as binding could be blocked by pretreating iDCs with AZN-D1 (Figure 2A). Analysis by CLSM revealed that hD1-KLH was rapidly internalized by iDCs (Figure 2B). After one hour of incubation at 37°C, hD1-KLH could be detected in 74% of the iDCs (data not shown). In contrast to hD1-KLH, KLH itself did not bind to iDCs, but was internalized by iDCs following one hour of incubation at 37°C (Figure 2C). These findings demonstrate that internalization of KLH by DCs is not receptor mediated, and likely depends on macropinocytosis.
Binding of hD1 to DC-SIGN and internalization by DCs. (A) iDCs were treated with 10 μg/mL hD1 (gray shaded curve), 100 μg/mL AZN-D1 (open dotted curve), or pretreated with 100 μg/mL AZN-D1 followed by 10 μg/mL hD1 incubation (open solid curve), followed by incubation with an Alexa Fluor 647–labeled goat anti–human IgG antibody. Binding of hD1 was analyzed by flow cytometry. (B) iDCs were incubated with AZN-D1 (•), hD1 (○), or AZN-L19 (□) at 4°C for one hour, and transferred to 37°C. Cells were fixed at various time points, and stained with Alexa Fluor–labeled secondary antibodies with or without prior permeabilization. The mean fluorescence was determined by flow cytometric analysis, and the amount of internalized antibody was plotted as a percentage of the amount of total cell-associated antibody. Data represent experiments performed in triplicate ± SD. (C) Internalization of hD1 was confirmed by CSLM. iDCs were incubated with hD1, AZN-D1, or their isotype controls 5G1.1 and mouse IgG1 (mIgG) for one hour at 37°C. Cells were stained with Alexa Fluor 647–labeled secondary antibodies (blue), followed by microscopic analysis. The image represents the middle focal plane of the DCs, with iris set at 2 nm. Original magnification, ×600.
Binding of hD1 to DC-SIGN and internalization by DCs. (A) iDCs were treated with 10 μg/mL hD1 (gray shaded curve), 100 μg/mL AZN-D1 (open dotted curve), or pretreated with 100 μg/mL AZN-D1 followed by 10 μg/mL hD1 incubation (open solid curve), followed by incubation with an Alexa Fluor 647–labeled goat anti–human IgG antibody. Binding of hD1 was analyzed by flow cytometry. (B) iDCs were incubated with AZN-D1 (•), hD1 (○), or AZN-L19 (□) at 4°C for one hour, and transferred to 37°C. Cells were fixed at various time points, and stained with Alexa Fluor–labeled secondary antibodies with or without prior permeabilization. The mean fluorescence was determined by flow cytometric analysis, and the amount of internalized antibody was plotted as a percentage of the amount of total cell-associated antibody. Data represent experiments performed in triplicate ± SD. (C) Internalization of hD1 was confirmed by CSLM. iDCs were incubated with hD1, AZN-D1, or their isotype controls 5G1.1 and mouse IgG1 (mIgG) for one hour at 37°C. Cells were stained with Alexa Fluor 647–labeled secondary antibodies (blue), followed by microscopic analysis. The image represents the middle focal plane of the DCs, with iris set at 2 nm. Original magnification, ×600.
Foreign antigens are taken up by DCs and shuttled, via endosomes, to the lysosomal compartment where they can be processed. In order to establish whether hD1-KLH reaches the lysosomal compartment, and to study uptake-kinetics and routing of KLH and D1-KLH, these proteins were directly labeled with Alexa Fluor 488 dye. Live imaging by CLSM revealed rapid uptake of hD1-KLH and KLH by iDCs. Both hD1-KLH and KLH were detected in the lysosomal compartment within one hour after their addition to the culture medium (Figure 2D; Supplemental Videos S1 and S2; see the Supplemental Videos link at the top of the online article, at the Blood website).
Induction of KLH responses by DC vaccination
In order to compare the antigen-presentation capacity of DCs pulsed with KLH to that of DCs targeted by hD1-KLH, we required KLH-specific T cells. To this end, PBLs were isolated from melanoma patients participating in a vaccination study. This study involved vaccination of patients with autologous DCs pulsed ex vivo with KLH and melanoma-associated antigens. Monocyte-derived DCs and PBLs from patients showing humoral as well as cellular responses against KLH were used for the targeting studies.
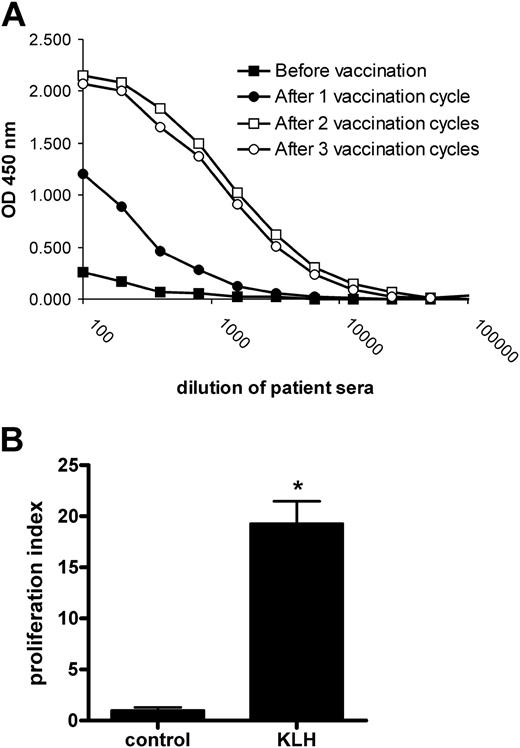
All 31 vaccinated patients participating in the clinical vaccination trial showed responses against KLH (data not shown). Humoral responses were readily detected in the patients' serum. KLH-specific IgGs were present after the first vaccination cycle, and their serum levels increased upon the second vaccination (Figure 3A). Cellular responses were analyzed using PBMCs obtained from patients one week before the start of the third vaccination cycle. Typically, PBMCs pulsed with KLH showed an increased proliferative response compared with unpulsed PBMCs, revealing the presence of KLH-reactive T cells in peripheral blood (Figure 3B). These findings show that KLH-pulsed mDCs effectively induced both humoral and cellular responses in vivo.
Binding and uptake of hD1-KLH by DCs. (A) iDCs were treated with 10 μg/mL hD1-KLH, 100 μg/mL AZN-D1, or pretreated with 100 μg/mL AZN-D1 followed by 10 μg/mL hD1 incubation, followed by incubation with an Alexa Fluor 647–labeled goat anti–human IgG antibody. Binding of hD1-KLH was analyzed by flow cytometry. (B) Internalization of hD1-KLH was confirmed by CSLM. iDCs were incubated with Alexa Fluor 647–labeled hD1-KLH (blue) for one hour at 37°C, followed by microscopic analysis. The image represents the middle focal plane of the DCs, with iris set at 2 nm. Original magnification, ×600. (C) iDCs were incubated with Alexa Fluor 488–labeled KLH for one hour at 4°C or 37°C. Subsequently, cells were fixed and analyzed by flow cytometric analysis. (D) iDCs were labeled with LysoTracker Red, followed by addition of Alexa Fluor 488–labeled (green) hD1-KLH or KLH. Cells were imaged by CSLM. Data represent bright field and corresponding fluorescent images of cells at various time points after addition of hD1-KLH or KLH to the culture medium. Original magnification, ×630.
Binding and uptake of hD1-KLH by DCs. (A) iDCs were treated with 10 μg/mL hD1-KLH, 100 μg/mL AZN-D1, or pretreated with 100 μg/mL AZN-D1 followed by 10 μg/mL hD1 incubation, followed by incubation with an Alexa Fluor 647–labeled goat anti–human IgG antibody. Binding of hD1-KLH was analyzed by flow cytometry. (B) Internalization of hD1-KLH was confirmed by CSLM. iDCs were incubated with Alexa Fluor 647–labeled hD1-KLH (blue) for one hour at 37°C, followed by microscopic analysis. The image represents the middle focal plane of the DCs, with iris set at 2 nm. Original magnification, ×600. (C) iDCs were incubated with Alexa Fluor 488–labeled KLH for one hour at 4°C or 37°C. Subsequently, cells were fixed and analyzed by flow cytometric analysis. (D) iDCs were labeled with LysoTracker Red, followed by addition of Alexa Fluor 488–labeled (green) hD1-KLH or KLH. Cells were imaged by CSLM. Data represent bright field and corresponding fluorescent images of cells at various time points after addition of hD1-KLH or KLH to the culture medium. Original magnification, ×630.
Humoral and cellular responses against KLH in patients vaccinated with KLH-pulsed DCs. (A) Patient serum was obtained both before the first, and after the first, second, and third vaccination cycles. Total IgG antibodies specific for KLH were detected by ELISA. Data represent optical density (OD) 450 values of serially diluted serum samples for a representative patient. (B) Patient PBMCs were isolated one week prior to the third vaccination cycle and cultured in the absence (control) or presence of 10 μg/mL KLH (KLH). Cellular responses were assessed in a tritiated thymidine incorporation assay. Data represent mean ± SD of experiments performed in 6-fold for a representative patient. Significant difference from control according to Student t test: *P < .001.
Humoral and cellular responses against KLH in patients vaccinated with KLH-pulsed DCs. (A) Patient serum was obtained both before the first, and after the first, second, and third vaccination cycles. Total IgG antibodies specific for KLH were detected by ELISA. Data represent optical density (OD) 450 values of serially diluted serum samples for a representative patient. (B) Patient PBMCs were isolated one week prior to the third vaccination cycle and cultured in the absence (control) or presence of 10 μg/mL KLH (KLH). Cellular responses were assessed in a tritiated thymidine incorporation assay. Data represent mean ± SD of experiments performed in 6-fold for a representative patient. Significant difference from control according to Student t test: *P < .001.
Targeting of KLH to DC-SIGN results in presentation of KLH antigen epitopes
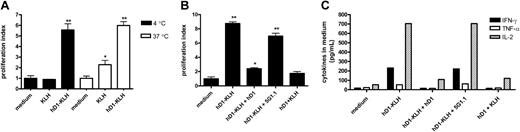
To demonstrate that antibody-mediated targeting of antigen to DC-SIGN leads to antigen presentation, autologous iDCs were incubated with 5 μg/mL KLH (± 0.63 nM) or hD1-KLH (± 0.53 nM) at 4°C for one hour, washed to remove unbound protein, and added to PBLs. Presentation of KLH epitopes to specific T cells was determined by measuring proliferative responses (Figure 4A-B) and the levels of cytokines secreted in the medium (Figure 4C). As endocytotic processes are inhibited at 4°C, and KLH does not bind to iDCs (Figure 2C), hD1-KLH can be internalized only after binding to DC-SIGN. iDCs incubated with hD1-KLH induced cellular responses by the PBLs, whereas iDCs incubated with KLH did not (Figure 4A-C), showing that targeting of KLH to DC-SIGN results in antigen presentation. We have previously demonstrated that the clinical vaccination protocol used in our studies results in a Th1-type immune response.20 Indeed, stimulation of patient PBLs with hD1-KLH–treated iDCs resulted in enhanced secretion of IL-2, IFN-γ, and TNF-α (Figure 4C), while IL-4, IL-5, and IL-10 levels were not detectable (data not shown). As expected, iDCs incubated with KLH at 37°C induced proliferative responses, as did iDCs incubated with hD1-KLH (Figure 4A).
Antibody-mediated targeting of antigen to DC-SIGN results in antigen presentation. (A) iDCs were incubated with medium (control), 5 μg/mL KLH, or 5 μg/mL hD1-KLH for one hour at 4°C or 37°C. Subsequently, iDCs were washed and cocultured with autologous KLH-responsive PBLs, derived from the patients enrolled in the vaccination trial. After 4 days, cellular responses were assessed in a proliferation assay. Data are mean proliferation indices relative to medium control for experiments performed in triplicate ± SD. Significant difference from medium control according to analysis of variance (ANOVA) and Bonferroni test: *P < .05; **P < .001. (B) iDCs were incubated with medium (control), 5 μg/mL hD1-KLH, 5 μg/mL hD1-KLH and 100 μg/mL hD1 (hD1-KLH + hD1), 5 μg/mL hD1-KLH and 100 μg/mL 5G1.1 (hD1-KLH + 5G1.1), or 5 μg/mL hD1 and 5 μg/mL KLH (hD1 + KLH) for one hour at 4°C. Subsequently, iDCs were washed and cocultured with autologous KLH-responsive PBLs, derived from patients enrolled in the vaccination trial. After 4 days, cellular responses were assessed in a proliferation assay. Data are mean proliferation indices relative to medium control for experiments performed in triplicate ± SD. Significant difference from medium control according to ANOVA and Dunnett test: *P < .05; **P < .01. (C) Production of IFN-γ, TNF-α, and IL-2 in the coculture experiment described in panel B. After 16 hours of coculturing iDCs and PBLs, supernatants were taken and cytokine levels were determined. Data represent cytokine levels in pooled samples of experiments performed in triplicate.
Antibody-mediated targeting of antigen to DC-SIGN results in antigen presentation. (A) iDCs were incubated with medium (control), 5 μg/mL KLH, or 5 μg/mL hD1-KLH for one hour at 4°C or 37°C. Subsequently, iDCs were washed and cocultured with autologous KLH-responsive PBLs, derived from the patients enrolled in the vaccination trial. After 4 days, cellular responses were assessed in a proliferation assay. Data are mean proliferation indices relative to medium control for experiments performed in triplicate ± SD. Significant difference from medium control according to analysis of variance (ANOVA) and Bonferroni test: *P < .05; **P < .001. (B) iDCs were incubated with medium (control), 5 μg/mL hD1-KLH, 5 μg/mL hD1-KLH and 100 μg/mL hD1 (hD1-KLH + hD1), 5 μg/mL hD1-KLH and 100 μg/mL 5G1.1 (hD1-KLH + 5G1.1), or 5 μg/mL hD1 and 5 μg/mL KLH (hD1 + KLH) for one hour at 4°C. Subsequently, iDCs were washed and cocultured with autologous KLH-responsive PBLs, derived from patients enrolled in the vaccination trial. After 4 days, cellular responses were assessed in a proliferation assay. Data are mean proliferation indices relative to medium control for experiments performed in triplicate ± SD. Significant difference from medium control according to ANOVA and Dunnett test: *P < .05; **P < .01. (C) Production of IFN-γ, TNF-α, and IL-2 in the coculture experiment described in panel B. After 16 hours of coculturing iDCs and PBLs, supernatants were taken and cytokine levels were determined. Data represent cytokine levels in pooled samples of experiments performed in triplicate.
Presentation of KLH epitopes by iDCs incubated with hD1-KLH at 4°C was abolished by preincubation of iDCs with the hD1 antibody, but was unaffected by preincubation with the isotype control 5G1.1 (Figure 4B-C). This confirms that hD1-KLH targeted specifically to DC-SIGN, resulting in presentation of KLH epitopes. Cross-linking of hD1 to KLH was required for induction of DC-SIGN–mediated presentation of KLH epitopes, since iDCs incubated with both KLH and hD1 antibody did not induce cellular responses (Figure 4B-C).
Targeting KLH to DC-SIGN enhances its immunogenicity
To determine whether DC-SIGN targeting increases the efficiency of antigen presentation, immature monocyte-derived DCs from previously immunized patients were incubated at 37°C with various concentrations of KLH or hD1-KLH. Subsequently, iDCs were washed, and a portion of the iDCs was matured with LPS. hD1-KLH by itself did not induce maturation of iDCs (data not shown). Both hD1-KLH–treated iDCs (Figure 5A) and mDCs (Figure 5B) induced cellular responses at 100-fold lower concentrations than KLH-pulsed DCs. Thus, antigen targeting via DC-SIGN significantly enhances immunogenicity.
KLH epitopes are presented up to 4 days after targeting DC-SIGN
After antigen uptake in the periphery, DCs migrate to the draining lymph nodes to present the processed antigen to T cells. It is imperative that DCs present the antigens over a prolonged period of time to initiate a significant immune response. We evaluated the efficiency of antigen presentation over time following targeting by incubating patient PBLs with autologous iDCs that had been pulsed with KLH or hD1-KLH 0, 2, or 4 days earlier. The results demonstrate that DCs targeted with hD1-KLH presented KLH epitopes up to 4 days after targeting. Moreover, at all time points evaluated, DCs targeted with hD1-KLH induced stronger proliferative responses than DCs pulsed with KLH (Figure 6).
Targeting of antigen to DC-SIGN enhances its immunogenicity. (A) iDCs were incubated with medium, 5 μg/mL KLH, or 5 μg/mL hD1-KLH for one hour at 37°C. Subsequently, iDCs were washed and cocultured with autologous KLH-responsive PBLs, derived from patients enrolled in the vaccination trial. After 4 days, cellular responses were assessed in a proliferation assay. Data are mean proliferation indices relative to medium control for experiments performed in triplicate ± SD. (B) Experiment performed as described in panel A, except that antigen-treated iDCs were matured with LPS before addition of PBLs.
Targeting of antigen to DC-SIGN enhances its immunogenicity. (A) iDCs were incubated with medium, 5 μg/mL KLH, or 5 μg/mL hD1-KLH for one hour at 37°C. Subsequently, iDCs were washed and cocultured with autologous KLH-responsive PBLs, derived from patients enrolled in the vaccination trial. After 4 days, cellular responses were assessed in a proliferation assay. Data are mean proliferation indices relative to medium control for experiments performed in triplicate ± SD. (B) Experiment performed as described in panel A, except that antigen-treated iDCs were matured with LPS before addition of PBLs.
hD1-KLH targeted to DCs activates both major histocompatibility complex (MHC) class I– and II–restricted naive T cells recognizing KLH epitopes
To evaluate whether hD1-KLH, besides triggering recall responses, is able to activate naive T cells, hD1-KLH was tested in an autologous in vitro culture system. PBLs from a healthy donor were stimulated by repetitive coculturing with hD1-KLH–pulsed autologous DCs. IL-10 was added to the cultures to stimulate expansion of CD8+ T cells.25 After 5 rounds of stimulation, PBLs were cocultured with DCs pulsed with KLH or hD1 to evaluate KLH-specific proliferative responses. The results demonstrate that hD1-KLH–targeted DCs activated KLH-specific naive T cells (Figure 7). Presentation of KLH epitopes was mediated by MHC class I and class II, since both class I and class II blocking antibodies significantly reduced the proliferative response, while an antibody control did not. DCs pulsed with hD1 antibody did not induce proliferative responses by the PBLs (Figure 7), demonstrating that the T-cell response is specific for KLH epitopes rather than epitopes contained within the hD1 antibody.
Antigen targeted to DC-SIGN is presented for at least 4 days. iDCs were incubated with medium, 5 μg/mL KLH, or 5 μg/mL hD1-KLH for one hour at 37°C. Subsequently, iDCs were washed and cultured for 0, 2, or 4 days before addition of autologous KLH-responsive PBLs, derived from patients enrolled in the vaccination trial. Four days after addition of PBLs, cellular responses were assessed in a proliferation assay. Data are mean proliferation indices relative to medium control for experiments performed in triplicate ± SD. Significant difference from KLH according to ANOVA and Bonferroni test: *P < .05; **P < .01.
Antigen targeted to DC-SIGN is presented for at least 4 days. iDCs were incubated with medium, 5 μg/mL KLH, or 5 μg/mL hD1-KLH for one hour at 37°C. Subsequently, iDCs were washed and cultured for 0, 2, or 4 days before addition of autologous KLH-responsive PBLs, derived from patients enrolled in the vaccination trial. Four days after addition of PBLs, cellular responses were assessed in a proliferation assay. Data are mean proliferation indices relative to medium control for experiments performed in triplicate ± SD. Significant difference from KLH according to ANOVA and Bonferroni test: *P < .05; **P < .01.
hD1-KLH–treated DCs activate naive T cells recognizing KLH epitopes. iDCs derived from a healthy donor were incubated with medium (control), 10 μg/mL KLH, or 10 μg/mL hD1 for one hour at 37°C. Subsequently, cells were washed and matured with LPS. mDCs were cocultured with autologous PBLs that had been repeatedly stimulated with DCs treated with hD1-KLH as described in “Patients, materials, and methods.” Some cocultures of KLH-pulsed mDCs and PBLs were supplemented with w6/32 antibody (KLH + class I block), IVA-12 (KLH + class II block), both w6/32 and IVA-12 (KLH + class I + class II block), or total mouse IgG (KLH + antibody control). Data are mean proliferation indices relative to medium control for experiments performed in triplicate ± SD. Significant difference according to ANOVA, followed by the Student-Newman-Keuls test: *P < .01; **P < .001.
hD1-KLH–treated DCs activate naive T cells recognizing KLH epitopes. iDCs derived from a healthy donor were incubated with medium (control), 10 μg/mL KLH, or 10 μg/mL hD1 for one hour at 37°C. Subsequently, cells were washed and matured with LPS. mDCs were cocultured with autologous PBLs that had been repeatedly stimulated with DCs treated with hD1-KLH as described in “Patients, materials, and methods.” Some cocultures of KLH-pulsed mDCs and PBLs were supplemented with w6/32 antibody (KLH + class I block), IVA-12 (KLH + class II block), both w6/32 and IVA-12 (KLH + class I + class II block), or total mouse IgG (KLH + antibody control). Data are mean proliferation indices relative to medium control for experiments performed in triplicate ± SD. Significant difference according to ANOVA, followed by the Student-Newman-Keuls test: *P < .01; **P < .001.
Discussion
In the present study, we explored the capacity of the type II C-type lectin DC-SIGN to function as a target receptor for vaccination purposes. The results demonstrate that antibody-mediated targeting of antigen to human DC-SIGN was 100-fold more efficient than pulsing DCs with antigen, and that antigen epitopes were presented for at least 4 days after targeting.
A critical step in designing a suitable targeting construct is the choice of targeting antibody. Various antibodies directed against the same receptor might bind distinct receptor epitopes, thus influencing biologic outcome. For example, not all antibodies directed against DC-SIGN are internalized following binding,13 and the anti-MR antibody PAM-1 induces maturation of iDCs, in contrast to a different isotype-matched anti-MR antibody.26 The mouse antihuman DC-SIGN antibody AZN-D1 has been previously described to be internalized by DCs,13 a prerequisite for successful targeting. However, the use of murine antibodies in humans presents numerous problems, including a short half-life and high immunogenicity.27 We were able to graft the CDR of AZN-D1 hypervariable domains onto a human composite IgG2/IgG4 antibody without loss of binding characteristics. The resulting hD1 antibody exhibited a similar binding affinity for DC-SIGN as AZN-D1, and did not induce maturation of iDCs (data not shown). hD1 binding induced rapid internalization of DC-SIGN, while internalization did not result in a lasting down-regulation of DC-SIGN expression (data not shown), thus minimizing the effect a therapeutic intervention might have on the biologic function of DC-SIGN. hD1 rapidly targeted antigen to the DC lysosomal compartment resulting in antigen presentation, and did not induce hD1-specific proliferative responses in the naive T-cell stimulation experiments. Thus, humanization of AZN-D1 resulted in an effective targeting antibody, and will facilitate introduction into clinical trials.
Vaccination strategies aimed at inducing cytotoxic T-cell help, such as antitumor therapies, require antigen presentation in the context of MHC class I. In addition, the induction of CTL responses requires bystander CD4+ T-cell help.28 Targeting DCs with hD1-KLH resulted in the activation of naive T cells in the context of both MHC class I and II. Although the major route for presentation of exogenous antigens is via class II, presentation via class I can occur via the process of cross-presentation. This process provides internalized proteins access to cytosolic proteasomes and their derived peptides access to the endoplasmatic reticulum (ER)–based class I processing machinery.29 Particulate antigens taken up by phagocytosis have access to this machinery since phagosomes fuse with the ER soon after or during their formation.30 Particulate antigens are more efficiently cross-presented than soluble antigens.31,32 However, a recent study by Ackerman et al reveals that internalized soluble proteins can escape proteolysis and also gain access to the lumen of the ER.33 This might explain how antibody-mediated targeting of the type I C-type lectins MR7 and DEC-20510 results in class I responses. Although we cannot exclude the possibility that, besides receptor-mediated endocytosis, a portion of our chimeric protein was taken up via macropinocytosis, our current findings strongly suggest that antibody-mediated targeting of DC-SIGN results in cross-presentation.
The technique commonly used in current clinical trials to load DCs with antigens involves ex vivo incubation with MHC class I and II binding peptides. Other techniques involve loading with tumor lysates or apoptotic tumor cells, and the introduction of genetic material to drive expression of specific antigens by the DC itself.2 Ideally, vaccines should deliver antigens to the DCs in vivo. Antibody-mediated targeting of antigens to DC surface receptors to stimulate antigen presentation in vivo has been shown to be far more potent than immunization with antigen in complete Freund adjuvant or splenic DCs pulsed with antigen ex vivo.6 However, mere targeting of antigens to DCs is not sufficient for induction of immunity. Antibody-mediated targeting of antigen to DEC-205 in mice leads to tolerance, and coadministration of a DC maturation stimulus is required to induce immunity.6,10,11 These findings are consistent with DC-based vaccination studies in humans, showing that DC maturation is a prerequisite for induction of immunity.20 Vaccination strategies for transplantation, allergy, autoimmunity, and chronic inflammatory diseases could exploit the finding that targeted iDCs induce tolerance. However, strategies aimed at inducing immunity will require a combination of antigens and DC activation factors. Agents that have been shown to activate DCs in vivo include anti-CD40 antibody,11 α-galactosylceramide,34 and the Toll-like receptor ligands LPS35 and cytosine-phosphateguanosine (CpG) oligonucleotides.36 A better understanding of differences in DC subsets, their activation pathways, and antigen uptake receptors will provide the information necessary for development of effective vaccines.
In the present study, we used a neoantigen to study targeting efficiency. A major challenge in cancer therapy is to break immunologic tolerance to tumor-associated self-antigens. Tolerance can be the result of clonal deletion, active suppression of antigen-specific T cells by regulatory T cells, or inadequate activation stimuli provided by tumor cells upon antigen presentation, resulting in T-cell anergy.37 One way of breaking tolerance is by disruption of negative regulatory mechanisms directly at the T-cell level. Antibody-mediated blockade of the cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) breaks tolerance against moderately immunogenic tumors38 and improves the efficiency of tumor cell–based vaccines against poorly immunogenic tumors.39 Depletion of CD25+ regulatory T cells prior to blocking of CTLA-4 improves vaccine efficiency even further.40 A second way of breaking tolerance is to recruit DCs as potent APCs. DCs can overcome the heightened threshold of anergic T cells41 and restore responsiveness of tolerogenic tumor-specific T cells42 in vitro. Moreover, studies in mice demonstrate that DC-based vaccines can break tolerance against (tumor-associated) self-antigens,43-47 resulting in regression of established tumors.47 Thus, a combination of antibody-mediated strategies targeting tumor-associated antigens to DCs and strategies modulating regulatory mechanisms at the T-cell level might provide effective cancer therapies.
In conclusion, antibody-mediated targeting of antigens to DC surface receptors represents an exciting way to induce immune responses. Our results demonstrate efficient delivery of antigen to DCs via DC-SIGN, resulting in naive as well as recall responses by T cells. These data expose DC-SIGN as a promising target molecule for antibody-mediated antigen delivery to DCs.
Prepublished online as Blood First Edition Paper, May 5, 2005; DOI 10.1182/blood-2005-01-0318.
Supported by research funding from Alexion Pharmaceuticals to P.J.T., the Dutch Cancer Society (Grant AZN/KUN 95/910) to I.J.M.d.V., and the TIL-foundation and European Commission contracts 512074 and 503037 to C.G.F.
I.J.M.d.V. and K.G. contributed equally to this paper.
S.J.F., R.P.R., and D.W. are employed by Alexion Pharmaceuticals, whose potential product was studied in the present work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank the Microscopic Imaging Center (MIC) of the NCMLS for use of their facilities, the technicians of the NCMLS Tumor Immunology Department Clinical DC group for assistance, and Greg Stahl at the Brigham and Women's Hospital, Harvard Medical School, Boston, MA.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal