Comment on De Franceschi et al, page 1454
Erythrocyte dehydration has long been considered detrimental to cell survival. In their study of mice with hemolytic anemia due to defective cytoskeletons, De Francheschi and colleagues challenge this view and show that dehydration actually protects the cell against excessive hemolysis.
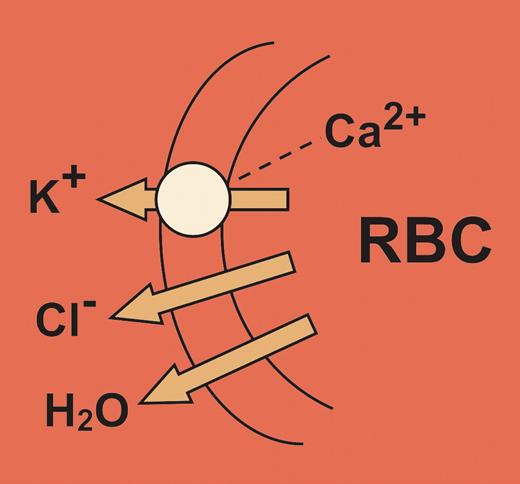
The opening of ionic channels allows massive fluxes of cations or anions to flow down their concentration gradients with effects that should clearly point to the channel function. However, the function of an unusual K+ channel, first described by George Gardos, a Hungarian hematologist many years ago,1 has remained elusive. Although dormant under normal physiologic conditions, this channel is opened by an increase in cytosolic-free Ca2+ concentration, which in turn produces a massive efflux of K+ ions from the erythrocyte with accompanying Cl loss and cellular dehydration (see figure). Activation of the Gardos channel may thus contribute to a number of pathologic states characterized by red cell dehydration and hemolysis, in particular hereditary spherocytosis and the formation of irreversibly sickled erythrocytes. In general, cellular dehydration has been thought to contribute to hemolysis, by reducing the deformability of the cell.
In this issue of Blood, De Franceschi and colleagues have examined the role of this Ca2+-activated K+ channel (termed KCNN4 in GenBank) in a laboratory mouse model of hereditary spherocytosis, the protein band 4.1–/– mouse. These gene-deleted mice have an erythrocyte cytoskeleton that is mechanically weakened so that the cell undergoes microvesiculation with loss of membrane surface area in the circulation.2 The 4.1–/– mice, like other genetic models with defective cytoskeletons (eg, spectrin–/– or protein 4.2–/–), have a brisk hemolytic anemia, a decreased mean corpuscular volume (MCV) and an increased mean cell hemoglobin concentration (MCHC). Kinetic analysis of the Gardos channel in the 4.1–/– erythrocytes showed a heightened sensitivity to activation by intracellular Ca2+ compared with wild-type erythrocytes. This led the authors to assess the function of the Gardos channel in vivo by administering clotrimazole, a channel blocker, to the mice. Paradoxically, the hemolytic parameters worsened, the red cell survival decreased, and some of the mice died. The surviving mice showed an increase in numbers of spherocytes and microspherocytes in the blood, indicating that without a functional Gardos channel, microvesiculation is much increased and hemolysis worsened in cells with defective cytoskeletons.
These findings force a reappraisal in our thinking about the role of K+ channels in spherocytosis. Dehydration is not the cause of the hemolysis but conversely loss of K+ and cellular dehydration is protective against excessive microvesiculation in cells with defective cytoskeletons. Further support comes from an earlier study in normal mice, which demonstrated that removal of erythrocyte surface area in vitro followed by reinfusion of the cells to the animal led to a decrease in cell volume presumably with dehydration.3 Erythrocytes are now known to undergo apoptosis at the end of their lifespans, although their apoptotic machinery differs from that of other cells. Loss of intracellular K+ is pivotal to the cell death program4 and Ca2+-activated K+ channels have been shown to contribute to the breakdown of phosphatidylserine asymmetry, which is the hallmark of apoptotic death of erythrocytes.5 Key questions remain. Does depletion of surface area activate the Gardos channel only when platelets are present in the same suspension? Do the microvesicles have exposed phosphatidylserine, which can present a prothrombotic surface, until they are cleared by the reticuloendothelial (RE) system? These studies on the laboratory mice from Bar Harbor, ME, may provide insights into the terminal events in the lifespan of the spherocyte. ▪FIG1
Calcium-activated potassium channel of erythrocytes showing the associated chloride and water loss from the cell.
Calcium-activated potassium channel of erythrocytes showing the associated chloride and water loss from the cell.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal