Comment on Tassone et al, page 1341
Tassone and colleagues present a new mouse model for the study of Waldenström macroglobulinemia.
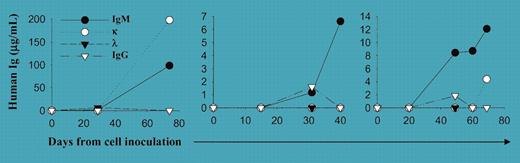
A major impediment to the understanding of Waldenström macroglobulinemia is the lack of adequate in vivo and in vitro models for the study of this indolent lymphoproliferative disorder. The bulk of studies describing disease biology and genetics have been descriptive because of the limited proliferative potential of the tumor cells.1 In this issue of Blood, Tassone and colleagues report on the first successful model that recapitulates Waldenström macroglobulinemia by implanting human xenograft cells into immunodeficient mice implanted with human fetal bone chips (SCID-hu). The investigators were initially unable to establish growth in the absence of the bone matrix. However, unlike the case of human multiple myeloma, where the SCID-hu model has been applied most successfully, the role of or need for human grafts in sustaining clonal growth derived from Waldenström macroglobulinemia bone marrow is not yet clear. Waldenström macroglobulinemia cells can grow in lymphoid organs and liver, although this only occurs in a minority of patients. Although further study is needed, this work presents the first bona fide model acceptable for preclinical testing of agents, as previously demonstrated in the application of rituximab (see figure). Furthermore, this model allows for the study of the complex interaction of clonal cells with other cells, such as mast cells, which are known to be commonly present in the bone marrow of patients with Waldenström macroglobulinemia. This is a big “mouse-sized” step for those who study Waldenström macroglobulinemia! ▪FIG1
Engraftment of primary patient WM cells in SCID-hu mice. See the complete figure in the article beginning on page 1341.
Engraftment of primary patient WM cells in SCID-hu mice. See the complete figure in the article beginning on page 1341.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal