Comment on Austin et al, page 1253
SBDS, the recently identified gene for Shwachman-Diamond syndrome (SDS), encodes a novel protein of unknown function. The protein, absent or diminished in cells from 6 of 7 patients with SDS, localizes to the nucleolus of normal fibroblasts in a cell-cycle–dependent manner.
Shwachman and Diamond described their eponymous syndrome in 1964 as an association of pancreatic insufficiency and bone marrow dysfunction.1 The syndrome (Online Mendelian Inheritance in Man [OMIM] entry 260400) is also characterized by short stature with delayed bone maturation and metaphyseal dysostosis; the liver, teeth, skin, and immune system may also be affected.2 Hematologic findings include neutropenia, combined with defects in neutrophil chemotaxis. Macrocytosis, hypoplastic anemia, and thrombocytopenia also occur frequently; 10% to 65% of patients have trilineage cytopenias.2 An increased risk of myelodysplastic syndrome and acute myelogenous leukemia is associated with this syndrome.2 FIG1
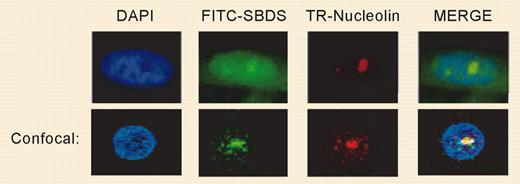
SBDS localizes to the nucleolus. See the complete figure in the article beginning on page 1253.
SBDS localizes to the nucleolus. See the complete figure in the article beginning on page 1253.
In 2003, Boocock and colleagues3 identified SDS-associated mutations in the previously uncharacterized SBDS gene, located adjacent to a 97% homologous pseudogene. Most SBDS mutations occur by gene conversion to a sequence encoding a truncated product. The predicted gene product is a highly conserved novel protein with unknown function, but the crystal structure and the association of Archaeal orthologs with RNA-processing genes suggest a role in RNA metabolism.3,4
In this issue of Blood, Austin and colleagues have now provided a rough idea of the Shwachman-Bodian-Diamond syndrome (SBDS) protein's intracellular location and, by implication, its function. The investigators raised a polyclonal antibody against a C-terminal peptide and used it for Western blot analyses and immunofluorescent imaging in cells from 7 patients with SDS and healthy controls. Not surprisingly, 4 patients with the common gene conversion mutation showed no immunoreactive SBDS protein and one patient with a splice site and one with a missense mutation showed markedly diminished levels. Importantly, one young patient with a classic phenotype had no detectable SBDS gene mutation and showed normal protein levels. Thus, as in other bone marrow failure syndromes with complex phenotypes, more than one gene may be responsible.
Immunofluorescent labeling of SBDS protein in normal fibroblasts and B-cell lines revealed striking localization to the nucleolus, with only small amounts in the cytoplasm. These data immediately suggest, along with the aforementioned gene homology, a role in ribosomal RNA processing. However, the nucleolar localization was cell-cycle dependent, with dispersion throughout the nucleus during S phase, a pattern suggestive of other nucleolar functions,5 most notably the sequestration of telomerase and of several proteins that control cell-cycle checkpoints. The multifaceted phenotype of SDS might reflect both intra- and nonnucleolar roles of SBDS. In fact, even the quintessential nucleolar protein, nucleolin, functions in different ways in multiple other locations.6
SDS shares involvement of the nucleolus and RNA processing with several related syndromes of bone marrow failure, 2 of which also feature short stature and skeletal abnormalities: cartilage-hair hypoplasia (OMIM 250250) derives from defects in the RNA component of RNase mitochondrial RNA processing, which is involved in nucleolar cleaving of pre-rRNA. X-linked dyskeratosis congenital (OMIM 305000) results from mutations in the gene encoding dyskerin, which associates with small nucleolar RNAs as well as with telomerase RNA. In addition, one gene identified as a cause of Diamond-Blackfan anemia (OMIM 105650) encodes the nucleolar-localized ribosomal protein S19. Future studies by Shimamura's laboratory and other groups studying these rare but important syndromes should help make “the rough places plane” in our understanding of the molecular basis of bone marrow failure, myelodysplasia, and leukemogenesis. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal