Abstract
Signaling by receptor activator of NF-κB (nuclear factor-κB) ligand (RANKL) is essential for differentiation of bone marrow monocyte-macrophage lineage (BMM) cells into osteoclasts. Here, we show RANKL stimulation of BMM cells transiently increased the intracellular level of reactive oxygen species (ROS) through a signaling cascade involving TNF (tumor necrosis factor) receptor-associated factor (TRAF) 6, Rac1, and NADPH (nicotinamide adenine dinucleotide phosphate) oxidase (Nox) 1. A deficiency in TRAF6 or expression of a dominant-interfering mutant of TRAF6 blocks RANKL-mediated ROS production. Application of N-acetylcysteine (NAC) or blocking the activity of Nox, a protein leading to the formation of ROS, with diphenylene iodonium (DPI) inhibits the responses of BMM cells to RANKL, including ROS production, activation of c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein (MAP) kinase, and extracellular signal-regulated kinase (ERK), and osteoclast differentiation. Moreover, both RANKL-mediated ROS production and osteoclast differentiation were completely blocked in precursors depleted of Nox1 activity by RNA interference or by expressing a dominant-negative mutant of Rac1. Together, these results indicate that ROSs act as an intracellular signal mediator for osteoclast differentiation.
Introduction
Receptor activator of NF-κB (nuclear factor-κB) ligand (RANKL; also called TRANCE [tumor necrosis factor (TNF) activation-induced cytokine], ODF [osteoclast differentiation factor], and OPGL [osteoprotegerin ligand])1-4 is a key factor stimulating the differentiation and activation of osteoclasts and, therefore, is essential for bone remodeling.5 The binding of RANKL to its receptor RANK leads to recruitment of TNF receptor-associated factor 6 (TRAF6) to the cytoplasmic domain of RANK, thereby resulting in the activation of distinct signaling cascades mediated by mitogen-activated protein (MAP) kinases, including c-Jun N-terminal kinase (JNK), p38 MAP kinase (p38), and extracellular signal-regulated kinase (ERK).6 It has been shown that JNK1-activated c-Jun signaling in cooperation with nuclear factor of activated T cells (NFAT) is key to RANKL-regulated osteoclast differentiation.7 In addition, stimulation of p38 results in the downstream activation of the mi/Mitf (microphthalmia/microphthalmia transcription factor), which controls the expression of the genes encoding tartrate-resistant acid phosphatase (TRAP) and cathepsin K, indicating the importance of p38 signaling cascades.6 Although our understanding of signaling pathways associated with osteoclast differentiation has advanced considerably recently, the mechanism of RANKL-mediated osteoclastogenesis, specifically the molecular linkage between TRAF6 and MAP kinases, is still unknown.
At high concentrations, reactive oxygen species (ROSs) cause oxidative stress that has been viewed as deleterious phenomena, including inflammatory response, apoptosis, or ischemia.8 Recent studies, however, indicate that small nontoxic amounts of ROS may play a role as a second messenger in the various receptor signaling pathways.9-13 Osteoclasts have shown to be activated by ROSs to enhance bone resorption,14 but little attention has been given to the role of ROSs in differentiation of macrophages and monocytes into osteoclasts. Signaling molecules such as JNK and p38, which are known to be essential for osteoclast differentiation,6,7 are sensitive to activation by ROSs.11,12 Thus, we hypothesized that signaling cascade(s) can be modulated by ROSs in bone marrow monocyte-macrophage lineage (BMM) cells.
Here, we show that RANKL generates ROSs in BMM cells. Examination of the mechanism by which RANKL generates ROSs revealed the involvement of TRAF6, Rac1, and NADPH (nicotinamide adenine dinucleotide phosphate) oxidase 1 (Nox1). These data suggest that RANKL-mediated ROS production serves to regulate RANKL signaling pathways, including JNK and p38 activation required for osteoclast differentiation.
Materials and methods
Reagents and plasmids
2′,7′-dichlorofluorescein diacetate (DCFH-DA) was purchased from Molecular Probes (Leiden, The Netherlands); all other chemicals and FLAG (five NH2-terminally deleted epitope-tagged) epitope (M2) were from Sigma-Aldrich (St Louis, MO); recombinant macrophage colony-stimulating factor (M-CSF) was from R&D Systems (Minneapolis, MN); RANKL was from PeproTech (Rocky Hill, NJ); and RANK-crystallizable fragment (Fc) was previously described.15 Polyclonal antibodies (Abs) specific for p38, phosphorylated p38 (Thr180/Tyr182), JNKs (p46 and p54), phosphorylated JNKs (Thr183/Tyr185), and monoclonal anti-phosphorylated p42/44 MAP kinase (ERKs) were purchased from New England Biolabs (Beverly, MA). TRAF6 (H-274) was from Santa Cruz Biotechnology (Santa Cruz, CA) and hemagglutinin (HA; 12CA5) was from Boehringer Mannheim (Mannheim, Germany). A monoclonal antibody (mAb) specific for Rac1 was from Transduction Laboratories (Lexington, KY).
Expression constructs encoding FLAG-tagged wild-type RANK, HA-tagged TRAF6 (amino acids [aa] 289-530), and maltose-binding protein (MBP)–RANKcyt, which comprises the cytoplasmic tail of RANK fused to MBP, were described.16 To generate a tetracycline-regulated FLAG-tagged RANK expression plasmid (pcDNA/TO-RANK), FLAG-tagged RANK was amplified by polymerase chain reaction (PCR) and inserted into pBluescriptSK+, and then subcloned into the EcoRI and XbaI sites of pcDNA/TO vector (Invitrogen, Carlsbad, CA). A retroviral vector, pMX-puro-TRAF6ΔZ3 was provided by Dr J. Inoue (Keio University, Kanagawa, Japan).
In vitro osteoclastogenesis
Nonadherent bone marrow–derived monocyte-macrophage cells derived from C57BL/6 mice were seeded and cultured in α-minimal essential medium (α-MEM) (GIBCO-BRL, Grand Island, NY) with 10% fetal bovine serum (FBS) containing 10 ng/mL M-CSF. After 2 days, adherent cells were used as BMM cells after washing out the nonadherent cells, including lymphocytes. TRAF6-deficient mice were described previously,17 and monocyte-macrophage progenitor cells of TRAF6-deficient mice were derived from the spleen and similarly cultured with 10 ng/mL M-CSF for 2 days. These osteoclast precursor cells were further cultured in the presence of 50 ng/mL soluble RANKL and 10 ng/mL M-CSF to generate osteoclasts. After 5 days, cells were fixed and stained for TRAP. TRAP-positive multinucleated (> 3 nuclei) cells were counted as osteoclast-like cells. The cells were observed with a Zeiss Axiovert 200 microscope equipped with a Plan-Neofluor 20×/0.50 objective lens (Carl Zeiss, Oberkochen, Germany), and images were obtained with an AxioCam HR (Carl Zeiss) using AxioVision 3.1 software (Carl Zeiss).
Cell stimulation, transfection, and analysis
Isolated BMM cells were extensively washed to remove exogenous growth factors, cultured in media with low serum (0.5% FBS, 6 hours), then stimulated by adding RANKL in the presence or absence of inhibitors as indicated. Inhibitors were added to cultures 60 minutes prior to addition of RANKL (50 ng/mL). After stimulation, the cells were washed in ice-cold phosphate-buffered saline, lysed, and subjected to Western blot analysis or immunoprecipitation as described in the next paragraph. 293 cells expressing inducible FLAG-tagged RANK were transfected by calcium phosphate precipitation.16 The cells were processed for analysis 24 hours after transfection. All cells were harvested and lysed in extraction buffer (20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) [pH 7.9], 150 mM NaCl, 1% Triton X-100, 10% glycerol, 5 mM EDTA (ethylenediaminetetraacetic acid), 1 mM Na3VO4, 1 mM phenylmethylsulfonylfluoride, 1 μg/mL leupeptin, 0.1 U/mL aprotinin) and cleared by centrifugation to obtain whole-cell extracts. Immunoprecipitations were performed on whole-cell extracts with anti-FLAG mAb. In brief, 1 μg Ab coupled to 30 μL protein G–Sepharose was incubated with 400 μg whole-cell extracts for 4 hours. The immunoprecipitates were then subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Maltose beads were used to precipitate the MBP-RANK fusion proteins (1 hour, 4°C) and subjected to SDS-PAGE as described earlier in this paragraph.
Assay of intracellular ROSs
Intracellular production of ROSs was assayed as described.10 In brief, at various times after stimulation with RANKL, dishes of confluent cells were washed with α-MEM lacking phenol red and then incubated in the dark for 5 minutes in Krebs-Ringer solution containing 5 μM DCFH-DA. Culture dishes were transferred to a Zeiss Axiovert 135 inverted microscope (Carl Zeiss) that was equipped with a 20 × Neofluor objective and Zeiss LSM 410 confocal attachment, and DCF (2′,7′-dichlorofluorescein) fluorescence was measured with an excitation wavelength of 488 nm and emission at 515-540 nm. To avoid photooxidation of DCF, the fluorescence image was collected by a single rapid scan (4-line average, total scan time of 4.33 seconds). After collection of the fluorescence image, the cells were imaged by digital interference contrast, and the mean relative fluorescence intensity for each group of cells was then measured by Carl Zeiss vision system (KS400, version 3.0).
Construction of 293 cells expressing RANK
The 293 cell line expressing FLAG-tagged RANK protein in an inducible manner was generated with use of a tetracycline-regulated gene expression system (Tet-ON gene expression system, T-REX system; Invitrogen). 293 cells were cotransfected with a regulatory plasmid, pcDNA6/TR (Blasticidin resistant) and the inducible expression plasmid encoding RANK, pcDNA/TO-RANK (Zeocin resistant). Cells were selected in culture medium containing 125 μg/mL Zeocin and 3 μg/mL Blasticidin. Zeocin- and Blasticidin-resistant clones were examined for expression of the FLAG-tagged RANK by Western blotting using anti-FLAG Ab.
Adenoviral infection
An adenovirus containing human catalase (Adcat) or green fluorescent protein (GFP; AdGFP), a generous gift of S. G. Rhee (National Institute of Health, Bethesda, MD), was prepared as previously described.18 The recombinant adenoviral-expressing vector encoding the catalase and the control vector were used to infect BMM cells. The infection efficiency was monitored by the expression of GFP.
Retroviral infection
To generate infectious retroviral particles, Plat-E cells (a kind gift from T. Kitamura, University of Tokyo, Tokyo, Japan) were transfected with the retroviral vectors, and supernatant collected from 24 to 48 hours after transfection was used as the viral stocks. For retrovirus infection, RAW264.7 or RAW-RacN17 cells (provided by Dr J. Kim, Korea University, Seoul, Korea), stably expressing a dominant-negative form of Rac1, were incubated with retrovirus stock with polybrene (4 μg/mL) for 6 hours. Two days after the exposure to virus, samples of the infected cells were assayed for infection efficiency, and the rest of the cells were further cultured in the presence or absence of RANKL for the osteoclast formation assay.
Semiquantitative RT-PCR
Reverse transcription (RT)–PCR was performed as described.2 Nucleotide sequences of PCR primers are as follows: Nox1 forward, 5′-AAGTGGCTGTACTGGTTGG-3′; Nox1 reverse, 5′-GTGAGGAAGAGTCGGTAGTT-3′; Nox2 forward, 5′-ACTTCTTGGGTCAGCACTGG-3′; Nox2 reverse, 5′-ATTCCTGTCCAGTTGTCTTCG-3′; Nox3 forward, 5′-CAGGCTCAAATGGACGGAAAGG-3′; Nox3 reverse, 5′-CCATGCCAATGCGGAACCCAGA-3′; Nox4 forward, 5′-CCTTGAACTGAATGCAGCAA-3′; Nox4 reverse, 5′-ACCACCTGAAACATGCAACA-3′; β-actin forward, 5′-TCACCCACACTGTGCCCATCTAC; and β-actin reverse, 5′-GAGTACTTGCGCTCAGGAGGAGC-3′.
siRNA synthesis and transfections
Duplex, small interfering (si) RNA oligonucleotides specific for murine Nox1 or Nox2 and a nonspecific control were synthesized using Silencer siRNA construction kit (Ambion, Austin, TX) with the following siRNA-encoding DNA oligonucleotides: Nox1 antisense, 5′-AACAACAGCACTCACCAATGCcctgtctc-3′; Nox1 sense, 5′-aaGCATTGGTGAGTGCTGTTGcctgtctc-3′; Nox2 antisense, 5′-AAGATGCCTGGAAACTACCTAcctgtctc-3′; Nox2 sense, 5′-aaTAGGTAGTTTCCAGGCATCcctgtctc-3′; control antisense, 5′-AAGACTCTTCTCTGTTCCAAGcctgtctc-3′; and control sense, 5′-aaCTTGGAACAGAGAAGAGTCcctgtctc-3′ (the 8 italicized nucleotide sequences at the 3′ end are complementary to the T7 promoter primer provided with the Silencer siRNA construction kit, and siRNA-producing sequences are capitalized). siRNAs were transfected into BMM cells using X-tremeGENE siRNA transfection reagent (Roche, Indianapolis, IN) according to the manufacturer's recommendations. After 48 hours, cultures were used for RT-PCR analysis. Transfection efficiency (usually > 95%) was assessed in parallel wells by using Silencer siRNA labeling kit (Mirus, Madison, WI).
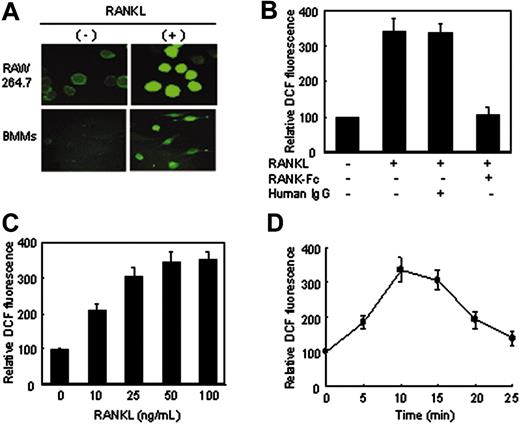
RANKL-induced ROS production in RAW264.7 cells and BMM cells. (A) RAW264.7 cells and BMM cells were preloaded with DCFH-DA and treated with RANKL (50 ng/mL) for 10 minutes. DCF fluorescence was measured with a confocal laser-scanning microscope as described in “Assay of intracellular ROSs.” Representative microscopic fields are shown. (B) A soluble form of RANK-Fc inhibits ROS production. BMM cells were pretreated for 60 minutes with 10 μg/mL human immunoglobulin G (IgG) or 5 μg/mL RANK-Fc, then stimulated for 10 minutes with RANKL (50 ng/mL). The production of ROSs was assayed as in panel A. Data are expressed relative to the value for nonstimulated cells. (C-D) BMM cells were stimulated with increasing concentrations of RANKL for 10 minutes (C) or with 50 ng/mL RANKL for different time intervals (D), and the production of ROSs was assayed as in panel A. Results are representative of at least 3 independent sets of similar experiments (A-D). Data are expressed as means ± SDs of triplicates.
RANKL-induced ROS production in RAW264.7 cells and BMM cells. (A) RAW264.7 cells and BMM cells were preloaded with DCFH-DA and treated with RANKL (50 ng/mL) for 10 minutes. DCF fluorescence was measured with a confocal laser-scanning microscope as described in “Assay of intracellular ROSs.” Representative microscopic fields are shown. (B) A soluble form of RANK-Fc inhibits ROS production. BMM cells were pretreated for 60 minutes with 10 μg/mL human immunoglobulin G (IgG) or 5 μg/mL RANK-Fc, then stimulated for 10 minutes with RANKL (50 ng/mL). The production of ROSs was assayed as in panel A. Data are expressed relative to the value for nonstimulated cells. (C-D) BMM cells were stimulated with increasing concentrations of RANKL for 10 minutes (C) or with 50 ng/mL RANKL for different time intervals (D), and the production of ROSs was assayed as in panel A. Results are representative of at least 3 independent sets of similar experiments (A-D). Data are expressed as means ± SDs of triplicates.
Rac activity assay
Cell lysates (150 μg) obtained from either RAW264.7 or RAW-RacN17 cells with or without stimulation with RANKL were incubated with 15 μg recombinant GST-PBD (glutathione S-transferase–p21-binding domain; human Pak1 aa 67-150) for 1 hour at 4°C, and then Rac activity was assayed as described.19
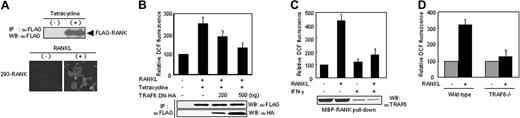
TRAF6 is required for RANKL-induced ROS production. (A) Characterization of tetracycline-regulated RANK-expressing 293 cells (293-RANK). Cell lysates (50 μg total protein) from cultures with (+) or without (–) 1 μg/mL tetracycline for 8 hours were prepared and incubated with anti-FLAG mAb. Immunoprecipitates (IPs) were analyzed by Western blotting (WB) to detect FLAG-RANK (top). 293-RANK cells induced with tetracycline were incubated for 10 minutes with (+) or without (–) RANKL (50 ng/mL), after which DCF fluorescence was measured as described in Figure 1A (bottom). (B) Effects of TRAF6.DN on RANKL-induced ROS production in 293-RANK cells. 293-RANK cells were transfected with expression constructs for TRAF6.DN (289-530)–HA as indicated. After 24 hours, the transfected cells were treated with tetracycline and further incubated for 8 hours, and then stimulated with RANKL (50 ng/mL) for 10 minutes. The production of ROSs was assayed as in Figure 1A. Immunoprecipitates were analyzed by Western blotting to detect FLAG-tagged RANKL and HA-tagged TRAF6.DN. (C) Effect of interferon-γ (IFN-γ) on ROS production. BMM cells were pretreated with 100 U/mL IFN-γ for 24 hours, and then stimulated with RANKL (50 ng/mL) for 10 minutes. The production of ROSs was assayed on the basis of DCF fluorescence as in Figure 1A. The level of TRAF6 was detected by pull-down with MBP-RANK fusion proteins (2 μg each) followed by Western blotting with polyclonal TRAF6 Ab. (D) Impairment for ROS production in splenic osteoclast precursors from TRAF6–/– mice. Wild-type or TRAF6–/– splenocytes were cultured in the presence of 10 ng/mL M-CSF, and then incubated for 10 minutes with (+; ▪) or without (–; ▦) RANKL (50 ng/mL). The production of ROSs was assayed as in Figure 1A. Results are representative of at least 3 independent sets of similar experiments (A-D). Data are expressed as means ± SDs of triplicates.
TRAF6 is required for RANKL-induced ROS production. (A) Characterization of tetracycline-regulated RANK-expressing 293 cells (293-RANK). Cell lysates (50 μg total protein) from cultures with (+) or without (–) 1 μg/mL tetracycline for 8 hours were prepared and incubated with anti-FLAG mAb. Immunoprecipitates (IPs) were analyzed by Western blotting (WB) to detect FLAG-RANK (top). 293-RANK cells induced with tetracycline were incubated for 10 minutes with (+) or without (–) RANKL (50 ng/mL), after which DCF fluorescence was measured as described in Figure 1A (bottom). (B) Effects of TRAF6.DN on RANKL-induced ROS production in 293-RANK cells. 293-RANK cells were transfected with expression constructs for TRAF6.DN (289-530)–HA as indicated. After 24 hours, the transfected cells were treated with tetracycline and further incubated for 8 hours, and then stimulated with RANKL (50 ng/mL) for 10 minutes. The production of ROSs was assayed as in Figure 1A. Immunoprecipitates were analyzed by Western blotting to detect FLAG-tagged RANKL and HA-tagged TRAF6.DN. (C) Effect of interferon-γ (IFN-γ) on ROS production. BMM cells were pretreated with 100 U/mL IFN-γ for 24 hours, and then stimulated with RANKL (50 ng/mL) for 10 minutes. The production of ROSs was assayed on the basis of DCF fluorescence as in Figure 1A. The level of TRAF6 was detected by pull-down with MBP-RANK fusion proteins (2 μg each) followed by Western blotting with polyclonal TRAF6 Ab. (D) Impairment for ROS production in splenic osteoclast precursors from TRAF6–/– mice. Wild-type or TRAF6–/– splenocytes were cultured in the presence of 10 ng/mL M-CSF, and then incubated for 10 minutes with (+; ▪) or without (–; ▦) RANKL (50 ng/mL). The production of ROSs was assayed as in Figure 1A. Results are representative of at least 3 independent sets of similar experiments (A-D). Data are expressed as means ± SDs of triplicates.
Statistical analysis
Data are presented as means plus or minus SDs from at least 3 independent experiments. Statistical significance was determined using 1-way analysis of variance (ANOVA) followed by the Student t test (*P < .05, **P < .005).
Results
RANKL generates ROSs in BMM cells
We first examined whether ROS production is detectable in osteoclast precursors upon RANKL treatment. Intracellular production of ROSs in the cells was measured with the cell-permeant, oxidation-sensitive dye DCFH-DA by using laser-scanning confocal microscopy. Stimulation of both RAW264.7 cells and BMM cells resulted in an increase in the intensity of DCF fluorescence (Figure 1A), indicating oxidation by hydroxyl radicals such as H2O2.20 The addition of soluble RANK-Fc significantly inhibited RANKL-stimulated ROS production in BMM cells (Figure 1B), confirming that RANKL generates ROSs through its cognate receptor. RANKL induced ROS production in a dose-dependent manner in BMM cells (Figure 1C), and the amount of ROS rapidly increased to its maximum level around 10 minutes after RANKL addition and thereafter decreased toward its basal level (Figure 1D). Taken together, these data indicate that RANKL generates ROSs in osteoclast precursors.
Requirement of TRAF6 in RANKL-induced ROS production
In view of previous results demonstrating that the binding of TRAF6 to the cytoplasmic domain of RANK is critical for RANKL signaling and osteoclast differentiation,16,21,22 it is an interesting issue whether ROS production is linked to TRAF6 binding. To answer this question, we generated a 293 cell line termed 293-RANK inducibly expressing FLAG epitope-tagged RANK. Expression of FLAG-RANK protein was detected by Western blotting in 293-RANK, 8 hours after the addition of tetracycline, but not in the uninduced 293-RANK cells (Figure 2A, top). RANKL treatment after FLAG-RANK induction in this clone increased the intensity of DCF fluorescence (Figure 2A, bottom). However, overexpression of TRAF6.DN (aa 289-530), a dominant-interfering mutant of TRAF6, significantly decreased the level of ROS induction in a dose-dependent manner (Figure 2B). IFN-γ is known to induce a rapid degradation of TRAF6 (Figure 2C, bottom, lane 3) by activation of the ubiquitin-proteasome system.23 Consistently, treatment of RANKL in IFN-γ–treated cells prevented ROS production (Figure 2C, top), which might be caused by a decreased level of TRAF6 (Figure 2C, bottom, lane 2 versus 4). To further explore the importance of TRAF6 in ROS production, we compared ROS levels in TRAF6-null osteoclast precursors and wild-type cells. The ROS levels induced by RANKL were significantly reduced in TRAF6-null osteoclast precursors (Figure 2D). Collectively, these findings indicate that TRAF6 plays a key linkage role in ROS production by RANKL.
NAC blocks RANKL-induced ROS production, MAP kinase activation, and osteoclastogenesis
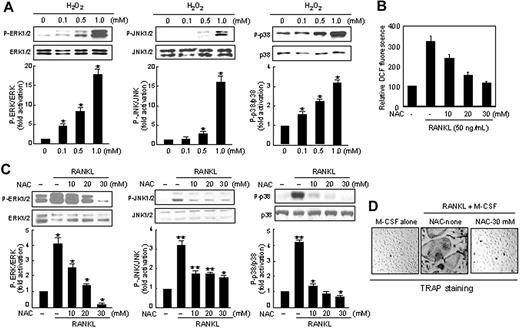
To correlate ROS production with RANKL responses, including MAP kinase activation and osteoclast differentiation, we first tested whether H2O2 alone activates MAP kinases in BMM cells. Activation of JNK, p38, or ERK was observed by treatment with H2O2 in a dose-dependent manner (Figure 3A). We next tested the effect of NAC, a chemical oxidant scavenger, on the RANKL responses. As expected, when cells were treated with N-acetylcysteine (NAC), RANKL-stimulated DCF fluorescence was significantly reduced in a dose-dependent manner (Figure 3B). In addition, the increasing amount of NAC exerted an inhibitory effect on RANKL-mediated ERK, JNK, or p38 MAP kinase activation (Figure 3C). We next examined the effect of a noncytotoxic concentration of NAC (30 mM) on osteoclast differentiation. NAC treatment strongly inhibited the formation of TRAP-positive osteoclasts with high numbers (> 3) of nuclei compared with that in the absence of NAC (Figure 3D). Taken together, these data show that the effect of NAC onROSs paralleled its effects on MAP kinase activation and osteoclast differentiation and imply that the elevated levels of ROSs might be the cause of MAP kinase activation, which in turn leads to stimulation of osteoclastogenesis.
Effects of N-acetylcysteine (NAC) on RANKL-induced ROS production, MAP kinase activation, and osteoclastogenesis. (A) BMM cells were treated with exogenous H2O2 in the indicated concentrations. Phosphorylated forms of ERK1/2 (P-ERK1/2), JNK1/2 (P-JNK1/2), and p38 MAP kinase (MAPK; P-p38) in whole-cell extracts were detected with phosphopeptide-specific Abs. The membranes were striped and probed with Abs against ERK1/2, JNK1/2, and p38 MAPK as indicated to confirm that similar amounts of whole-cell extracts were analyzed. Protein bands were quantified by densitometry, and levels of phosphorylated MAPK were normalized to levels of MAPK, respectively. The fold increase in the stimulated cells compared with untreated cells is shown. Data represent the means ± SDs of at least 3 independent experiments. *P < .05. (B) RAW264.7 cells were pretreated with increasing doses of NAC as indicated for 60 minutes. Cells were then treated with 50 ng/mL RANKL and further incubated for 10 minutes, and ROS was assayed as in Figure 1A. (C) As in panel A, except that NAC was used instead of H2O2. *P < .05; **P < .005. (D) Inhibitory effects of NAC on osteoclastogenesis in BMM cells. BMM cells were incubated with RANKL and M-CSF in the absence or presence of NAC (30 mM). On day 5, cells were fixed and stained for TRAP. TRAP-positive cells appeared as red cells (original magnification, × 100). Formation of TRAP-positive multinucleated cells (MNCs) was inhibited by NAC treatment.
Effects of N-acetylcysteine (NAC) on RANKL-induced ROS production, MAP kinase activation, and osteoclastogenesis. (A) BMM cells were treated with exogenous H2O2 in the indicated concentrations. Phosphorylated forms of ERK1/2 (P-ERK1/2), JNK1/2 (P-JNK1/2), and p38 MAP kinase (MAPK; P-p38) in whole-cell extracts were detected with phosphopeptide-specific Abs. The membranes were striped and probed with Abs against ERK1/2, JNK1/2, and p38 MAPK as indicated to confirm that similar amounts of whole-cell extracts were analyzed. Protein bands were quantified by densitometry, and levels of phosphorylated MAPK were normalized to levels of MAPK, respectively. The fold increase in the stimulated cells compared with untreated cells is shown. Data represent the means ± SDs of at least 3 independent experiments. *P < .05. (B) RAW264.7 cells were pretreated with increasing doses of NAC as indicated for 60 minutes. Cells were then treated with 50 ng/mL RANKL and further incubated for 10 minutes, and ROS was assayed as in Figure 1A. (C) As in panel A, except that NAC was used instead of H2O2. *P < .05; **P < .005. (D) Inhibitory effects of NAC on osteoclastogenesis in BMM cells. BMM cells were incubated with RANKL and M-CSF in the absence or presence of NAC (30 mM). On day 5, cells were fixed and stained for TRAP. TRAP-positive cells appeared as red cells (original magnification, × 100). Formation of TRAP-positive multinucleated cells (MNCs) was inhibited by NAC treatment.
To further confirm the relevance between ROS production and osteoclast differentiation, BMM cells were infected with catalase-expressing adenovirus or with a control virus. As expected, no effect on ROS production was observed with the control virus (data not shown). In contrast, expression of catalase in BMM cells blocked RANKL-induced ROS production (data not shown) and inhibited the formation of TRAP-positive osteoclasts in a dose-dependent manner (Figure 4), showing the involvement of ROSs in osteoclast differentiation.
NADPH oxidase inhibitor blocks RANKL-induced ROS production, MAP kinase activation, and osteoclastogenesis
The formation of ROSs in both phagocytic and nonphagocytic cells involves membrane-localized NADPH oxidases (Noxs).24 To determine whether an enzyme functionally similar to the NADPH oxidases is involved in RANKL-induced ROS production, we treated BMM cells with diphenylene iodonium (DPI), a specific inhibitor for flavoprotein that is a constituent of the NADPH oxidase complex. The addition of DPI abolished the rise in DCF fluorescence by RANKL treatment in a dose-dependent manner (Figure 5A). Likewise, DPI treatment blocked activation of JNK, p38, or ERK mediated by RANKL (Figure 5B) as well as RANKL-dependent osteoclastogenesis of BMM cells (Figure 5C). The fact that treatment of osteoclast precursors with DPI blocked RANKL responses supports the view that ROSs produced by an NADPH oxidase is required for osteoclast differentiation.
Suppressive effect of an antioxidant enzyme, catalase, on RANKL-induced osteoclastogenesis in BMM cells. (Top) BMM cells were infected with a recombinant adenovirus for catalase (AdCat) or the control adenovirus expressing GFP (AdGFP) with the indicated MOI (multiplicity of infection). On day 5, infected cells were stained for TRAP, and TRAP-positive MNCs were counted (bottom). Formation of TRAP-positive MNCs was severely suppressed in catalase virus-infected BMM cells (original magnification, × 100). Data represent means ± SDs of 3 independent experiments, each performed in triplicates.
Suppressive effect of an antioxidant enzyme, catalase, on RANKL-induced osteoclastogenesis in BMM cells. (Top) BMM cells were infected with a recombinant adenovirus for catalase (AdCat) or the control adenovirus expressing GFP (AdGFP) with the indicated MOI (multiplicity of infection). On day 5, infected cells were stained for TRAP, and TRAP-positive MNCs were counted (bottom). Formation of TRAP-positive MNCs was severely suppressed in catalase virus-infected BMM cells (original magnification, × 100). Data represent means ± SDs of 3 independent experiments, each performed in triplicates.
Inhibition of Nox1 by RNA interference suppresses RANKL-mediated ROS production and osteoclastogenesis
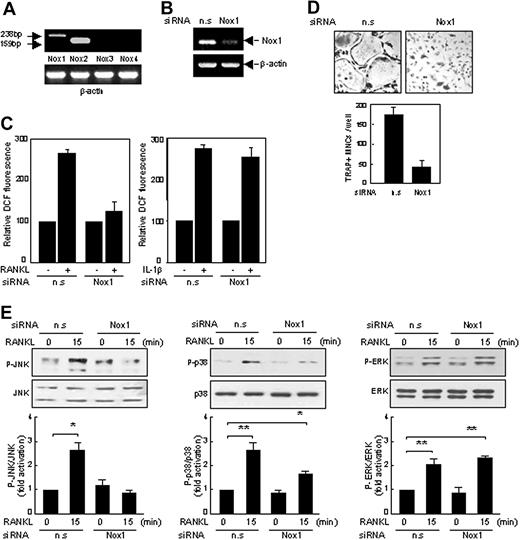
To find which Nox isozyme is responsible for the RANKL responses, we examined the expression of Nox isozymes in BMM cells with semiquantitative RT-PCR. As expected, Nox2, gp91phox, was found to be the main isotype expressed in BMM cells (Figure 6A). Interestingly, expression of Nox1 was also detectable at a low level in BMM cells, whereas expression of the other Nox members such as Nox3 and Nox4 was undetectable. To investigate the role of Nox1 in ROS production and osteoclastogenesis, we performed loss-of-function experiments using siRNAs. Nox1 was effectively knocked down by Nox1-specific siRNAs, as shown by RT-PCR (Figure 6B). Remarkably, the silencing of Nox1 in BMM cells resulted in a significant decrease in ROS production in response to RANKL stimulation (Figure 6C). Such reduction was not observed in IL-1β stimulation of BMM cells which also generates ROS production (Figure 6C). Likewise, siRNA of Nox1 blocked RANKL-mediated osteoclastogenesis (Figure 6D) as well as activation of JNK or p38 mediated by RANKL (Figure 6E). However, Nox2 knockdown had no effects on the ROS production and osteoclastogenesis induced by RANKL (data not shown). These results suggest that Nox1 is the critical mediator of the RANKL-mediated responses in BMM cells.
Suppressive effects of NADPH oxidase inhibitor DPI on ROS production, MAP kinase activation, and osteoclastogenesis in BMM cells. (A) BMM cells were pretreated with increasing doses of DPI as indicated for 60 minutes. Cells were then treated with 50 ng/mL RANKL and further incubated for 10 minutes, and ROS levels were determined as in Figure 1A. (B) As in Figure 3A, except that DPI was used instead of H2O2. *P < .05; **P < .005. (C) Suppressive effects of DPI on osteoclastogenesis in BMM cells. (Left) BMM cells were cultured with RANKL in the absence or presence of DPI for 5 days and stained for TRAP. (Right) TRAP-positive MNCs were counted. Formation of TRAP-positive MNCs was inhibited in a dose-dependent manner (original magnification, × 100). Data represent means ± SDs of 3 independent experiments, each performed in duplicate (A-C).
Suppressive effects of NADPH oxidase inhibitor DPI on ROS production, MAP kinase activation, and osteoclastogenesis in BMM cells. (A) BMM cells were pretreated with increasing doses of DPI as indicated for 60 minutes. Cells were then treated with 50 ng/mL RANKL and further incubated for 10 minutes, and ROS levels were determined as in Figure 1A. (B) As in Figure 3A, except that DPI was used instead of H2O2. *P < .05; **P < .005. (C) Suppressive effects of DPI on osteoclastogenesis in BMM cells. (Left) BMM cells were cultured with RANKL in the absence or presence of DPI for 5 days and stained for TRAP. (Right) TRAP-positive MNCs were counted. Formation of TRAP-positive MNCs was inhibited in a dose-dependent manner (original magnification, × 100). Data represent means ± SDs of 3 independent experiments, each performed in duplicate (A-C).
Expression of a dominant-negative mutant of Rac1 blocks ROS production and osteoclastogenesis
The small guanosine triphosphatase (GTPase) Rac1, predominant in macrophages and monocytes,25 is a cytosolic component of NADPH oxidase complex and is responsible for the activation of NADPH oxidases.26 To examine the involvement of Rac1 in RANKL-mediated ROS production and osteoclast differentiation, RAW264.7 and RAW-RacN17 cells stably expressing a dominant-negative form of Rac1 were used. As shown in Figure 7A, RANKL activated endogenous Rac1 in RAW264.7 cells but not in RAW-RacN17 cells, and this activation was greatly reduced by expression of a dominant-negative form of TRAF6 (T6ΔZ3), suggesting that the Rac1 activation by RANKL is dependent on TRAF6. Moreover, both ROS production (Figure 7B) and osteoclastogenesis (Figure 7C) were inhibited in RAW-RacN17 cells compared with its parental RAW264.7 cells. Collectively, these results indicate that RANKL-mediated ROS generation and osteoclastogenesis require Rac1.
Discussion
Although the production of ROSs by phagocytes such as macrophages and neutrophils has been mainly studied in the context of bacterial killing,8,24 our data imply that RANKL, which stimulates osteoclast differentiation, induced the production of ROSs for physiologic responses through RANKL-TRAF6 axis that is known to play a central role in osteoclastogenesis.6,21,22 We also found that the small GTPase Rac1 and Nox1, functioning downstream of TRAF6, are essential for RANKL-mediated ROS production and osteoclastogenesis. Moreover, our current findings suggest that RANKL-induced ROSs play a significant role in the activation of MAP kinases. Thus, these results suggest, besides their destructive properties in the microbicidal role, that ROSs produced in macrophages and monocytes through RANKL-TRAF6-Rac1-NADPH oxidase–dependent pathways may be involved in mediation of osteoclast differentiation.
Inhibition of RANKL responses by Nox1 siRNAs in BMM cells. (A) RT-PCR analysis of the expression of Nox isoforms in BMM cells. Expected product sizes of Nox 1 to 4 are 238 base pair (bp), 159 bp, 209 bp, and 220 bp, respectively. Expression of Nox1 and Nox2 mRNAs was indicated with arrows. n.s or Nox1-specific siRNAs were synthesized and transfected into BMM cells (B-E). (B) Specific reduction of Nox1 mRNA was shown by RT-PCR. (C) BMM cells transfected with nonspecific (n.s) or Nox1 siRNAs were treated with RANKL or interleukin 1β (IL-1β) for 10 minutes, and ROS levels were determined as in Figure 1A. (D) On day 5, cells were fixed and stained for TRAP. Formation of TRAP-positive MNCs was severely suppressed by Nox1 siRNAs (original magnification, × 100). (E) As in panel C, except that whole-cell extracts were detected with phosphopeptide-specific Abs as in Figure 3A. *P < .05; **P < .005. Data represent means ± SDs of 3 independent experiments, each performed in duplicate (C-E).
Inhibition of RANKL responses by Nox1 siRNAs in BMM cells. (A) RT-PCR analysis of the expression of Nox isoforms in BMM cells. Expected product sizes of Nox 1 to 4 are 238 base pair (bp), 159 bp, 209 bp, and 220 bp, respectively. Expression of Nox1 and Nox2 mRNAs was indicated with arrows. n.s or Nox1-specific siRNAs were synthesized and transfected into BMM cells (B-E). (B) Specific reduction of Nox1 mRNA was shown by RT-PCR. (C) BMM cells transfected with nonspecific (n.s) or Nox1 siRNAs were treated with RANKL or interleukin 1β (IL-1β) for 10 minutes, and ROS levels were determined as in Figure 1A. (D) On day 5, cells were fixed and stained for TRAP. Formation of TRAP-positive MNCs was severely suppressed by Nox1 siRNAs (original magnification, × 100). (E) As in panel C, except that whole-cell extracts were detected with phosphopeptide-specific Abs as in Figure 3A. *P < .05; **P < .005. Data represent means ± SDs of 3 independent experiments, each performed in duplicate (C-E).
TRAF6-dependent Rac1 activation regulates RANKL-mediated ROS production and osteoclastogenesis. (A) RAW264.7 and RAW-RacN17 cells (left) or RAW264.7 cells infected with control or mutant TRAF6 (pMX-T6ΔZ3) virus (right) were treated with RANKL for 5 minutes and lysed. The lysates were incubated with GST-PBD fusion protein that binds to GTP-bound Rac1. Proteins complexed to the beads were recovered by centrifugation, and the active GTP-Rac1 and Rac1 were detected with an anti-Rac1 antibody. *P < .05. (B) RAW264.7 and RAW-RacN17 cells were treated with RANKL for 10 minutes, and ROS levels were determined as in Figure 1A. (C) RAW264.7 and RAW-RacN17 cells were treated with RANKL for 5 days. (Left) The cells were fixed and stained for TRAP, and (right) TRAP-positive MNCs were counted. Formation of TRAP-positive MNCs was severely suppressed in RacN17 cells (original magnification, × 100). Data represent means ± SDs of 3 independent experiments, each performed in duplicate (A-C).
TRAF6-dependent Rac1 activation regulates RANKL-mediated ROS production and osteoclastogenesis. (A) RAW264.7 and RAW-RacN17 cells (left) or RAW264.7 cells infected with control or mutant TRAF6 (pMX-T6ΔZ3) virus (right) were treated with RANKL for 5 minutes and lysed. The lysates were incubated with GST-PBD fusion protein that binds to GTP-bound Rac1. Proteins complexed to the beads were recovered by centrifugation, and the active GTP-Rac1 and Rac1 were detected with an anti-Rac1 antibody. *P < .05. (B) RAW264.7 and RAW-RacN17 cells were treated with RANKL for 10 minutes, and ROS levels were determined as in Figure 1A. (C) RAW264.7 and RAW-RacN17 cells were treated with RANKL for 5 days. (Left) The cells were fixed and stained for TRAP, and (right) TRAP-positive MNCs were counted. Formation of TRAP-positive MNCs was severely suppressed in RacN17 cells (original magnification, × 100). Data represent means ± SDs of 3 independent experiments, each performed in duplicate (A-C).
We have shown that RANKL activates Rac1 in osteoclast precursors. Importantly, expression of a Rac1 dominant-negative mutant (Rac1N17) blocked ROS production and osteoclast differentiation induced by RANKL. It has been reported that the small GTPase Rac1 is a cytosolic component of NADPH oxidase complex and is responsible for the activation of an NADPH oxidase.26 In addition, Rac1 binds to Nox1 to stimulate its NADPH oxidase activity in a growth factor-dependent manner.27 Our findings are consistent with those reports in that RANKL signaling is linked to Rac1-Nox1 complex to generate ROSs. It is possible that similar mechanisms may control distinct biologic outcomes by growth and differentiation factors, despite their use in overlapping signaling cascades.
Recently, novel gp91phox (Nox2) homologues, termed Nox1, Nox3, Nox4, and Nox5, were identified in various nonphagocytic cells.24 Mature osteoclasts express an alternative oxidase, Nox4, also responsible for ROS generation.28 Our data from RT-PCR demonstrated that Nox1 is expressed in BMM cells, suggesting that it is Nox1 rather than Nox2 or Nox4 that is important for RANKL-induced ROS production in BMM cells. Indeed, Nox1 siRNAs resulted in an inhibition of both RANKL-induced ROS formation and osteoclastogenesis. There was a clear difference in the level of inhibition of RANKL-mediated ERK activation by DPI or by Nox1 siRNA. Specifically, DPI blockage of RANKL-induced ROSs resulted in a decrease in ERK activity, whereas Nox1 siRNA had no effect. This likely results from the specificity of Nox1 inhibition, which was revealed by Nox1 siRNA application. On the contrary, the most widely used inhibitor of NADPH oxidase, DPI, is a broad one, including the neuronal (nNOS) and endothelial (eNOS) nitric oxide isoforms29 and other flavo-containing proteins.30 It is worth noting that IL-1β stimulates osteoclast differentiation in the presence of RANKL in a synergistic fashion.31 In addition, it has been shown that IL-1β treatment in monocytes led to a production of ROSs which requires an NADPH oxidase.32 Unlike RANKL, IL-1β–mediated ROS production was not affected when Nox1-silenced BMM cells were stimulated by IL-1β, suggesting that distinct Nox isozymes regulate ROS production.
The mechanism by which ROSs activate MAP kinases is not completely understood. Note that the activation of MAP kinases by a number of stimuli, including growth factors and cytokines, appears to require ROSs,33,34 suggesting that the activation of MAP kinases by ROSs might be a rather common, if not general, response to different stimuli. Recently, it has been reported that ROS oxidize cysteine residues in proteins, including protein tyrosine phosphatases (PTPs; eg, PTEN [phosphatase tensin homologue deleted on chromosome 10]), thus inactivating the proteins.35 These results suggest that inhibition of tyrosine phosphatase activity by ROSs may account for another mechanism in triggering downstream signaling events. Therefore, it is possible that ROSs may regulate the activation of MAP kinases by inactivating a tyrosine phosphatase activity. In support of this possibility, we have preliminary results that RANKL stimulation induces diverse phosphotyrosyl proteins in RAW264.7 cells (data not shown), although there is no evidence that RANK has a functional tyrosine kinase domain. Pretreatment with NAC or DPI reduced RANKL-induced tyrosine phosphorylation, suggesting that the relationships between RANKL-induced ROSs and inhibition of PTP(s). Otherwise, we cannot rigorously rule out the possibility that serine-threonine phosphatases are regulated by ROSs, thereby affecting MAP kinases activation. Further study will be required to elucidate how RANKL-induced ROSs regulate the activation of MAP kinases directly or indirectly.
Recently, consistent with our data it has been reported that administration of NAC or ascorbate antioxidants decreased osteoclast number on bone surface, thereby abolishing ovariectomy-induced bone loss.36 In this model, ROSs have been implicated as a pathologic agent as in inflammation and aging. However, our results show that ROSs may act as a physiologic second messenger in RANKL signaling essential for osteoclastogenesis. We want to emphasize that a mechanism similar to the role of ROSs in osteoclast precursors is also operational during plant cell growth37 and invertebrate vulval development,38 indicating that ROSs can control cell development or differentiation.
In conclusion, our results indicate that ROSs produced by RANKL in BMM cells stimulate osteoclast differentiation, whereas decreasing the level of ROSs reverses RANKL responses. Therefore, application of ROS scavengers or inhibitors of RANKL-induced ROSs generating pathways may be beneficial strategies for an alternative therapy of bone diseases.
Prepublished online as Blood First Edition Paper, April 7, 2005; DOI 10.1182/blood-2004-09-3662.
Supported in part by Molecular and Cellular BioDiscovery Research Grant (M1-0401-00-0045) from the Ministry of Science and Technology (S.Y.L), the Korea Science and Engineering Foundation (KOSEF) through the Center for Cell Signaling Research at Ewha Womans University, the 21C Frontier Functional Proteomics Project (FPR02A7-32-110), and a Brain Korea 21 fellowship from the Ministry of Education (N.L. and S.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. Kim for critical reading of the manuscript, J. Inoue for providing the pMX-TRAF6 vector, and T. Kitamura for providing the Plat-E cell line.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal