Abstract
We report long-term results of treatment of myelodysplastic syndrome (MDS) with erythropoietin and granulocyte colony-stimulating factor (G-CSF). A total of 129 patients were followed up 45 months after last inclusion in the Nordic MDS Group studies. Erythroid response rate was 39% and median response duration 23 months (range, 3-116 months or more). Complete responders showed longer response duration than partial responders (29 versus 12 months, P = .006). The International Prognostic Scoring System (IPSS) groups Low/Intermediate-1 (Low/Int-1) had longer response duration than Int-2/High (25 versus 7 months, P = .002). The time until 25% developed acute myeloid leukemia (AML) was longer in the good and intermediate predictive groups for erythroid response compared with the poor predictive group (52 versus 13 months, P = .008). Only 1 of 20 long-term responders developed AML. We assessed the effect on long-term outcome by comparing treated patients with untreated patients selected from the IPSS database using multivariate Cox regression, adjusting for major prognostic variables. There was no difference in survival (odds ratio [OR], 0.9; 95% confidence interval [CI], 0.7-1.2; P = .55) or risk of AML evolution (OR, 1.3; 95% CI, 0.7-2.2; P = .40) between treated and untreated patients. Patients with high/intermediate probability of response and with IPSS Low/Int-1 show frequent and durable responses without adverse effects on outcome, while other patients should not be considered candidates for this treatment.
Introduction
Myelodysplastic syndrome (MDS) constitutes a group of malignant stem cell disorders characterized by ineffective hematopoiesis and peripheral cytopenia. The anemia is often severe, transfusion dependent, and associated with impaired quality of life.2
Two classifications of MDS are currently used, the French-American-British (FAB) classification3 from 1982 and the World Health Organization (WHO) classification4 from 2001. The most accurate prognostic information regarding survival and acute myeloid leukemia (AML) evolution is provided by the International Prognostic Scoring System (IPSS)5 developed by the International MDS Risk Analysis Workshop (IMRAW) in 1997. Based on percentage of bone marrow (BM) blasts, number of cytopenias, and karyotype, the IPSS stratifies patients into 4 risk categories: Low, Intermediate-1 (Int-1), Intermediate-2 (Int-2), and High.
There is accumulated evidence that erythropoietin (Epo) and granulocyte colony-stimulating factor (G-CSF) is an effective treatment for the anemia of MDS, with erythroid response rates of around 40% and a clear synergistic effect between the 2 drugs.2,6-8 We have developed a predictive model for erythroid response based on serum Epo (S-Epo) level and transfusion need, defining 3 groups of patients with good, intermediate, and poor erythroid response rate (74%, 23%, and 7%, respectively).2,9 Patients in the poor predictive group have an S-Epo level above 500 U/L and a transfusion need of 2 or more red blood cell (RBC) units per month.
We have demonstrated an antiapoptotic effect of G-CSF on MDS erythropoiesis, which is mediated via inhibition of mitochondrial release of cytochrome c.10,11 Epo is also a well-known inhibitor of apoptosis.12 These reports call for a certain degree of caution when EPO and G-CSF are used clinically for a clonal disorder such as MDS. Although our previous report6 showed no signs of increased blast proliferation after treatment, an extended and statistically more accurate analysis is warranted.
We have previously reported a median response duration for treatment with Epo and G-CSF (Epo-G) of around 2 years and that a response to treatment is associated with an improved quality of life (QoL).2,6,13 A recently published randomized study of 60 patients showed a significant effect of treatment on erythroid response but a shorter response duration than usually reported.6,8,14 A preliminary reported randomized phase 3 trial compared chronic administration of G-CSF with observation in 102 high-risk MDS patients.15 G-CSF–treated patients with refractory anemia with excess blasts (RAEB) had shorter survival than untreated RAEB patients after a period of follow-up that was relatively short. This difference was not caused by AML evolution.
The aim of this study was to analyze long-term outcome of a cohort of 129 MDS patients treated with Epo-G2,6,13 and to compare these patients with an untreated cohort from the IPSS/IMRAW database.5 Our results show that treatment with Epo-G can induce long-lasting responses and transfusion independency in defined subsets of MDS patients without influence on overall survival or risk of leukemic transformation.
Patients and methods
Patients
Three Nordic MDS Group studies on treatment of anemia in MDS with Epo-G were performed between 1990 and 1999.2,6,13 Inclusion criteria were a diagnosis of refractory anemia (RA), RA with ringed sideroblasts (RARS), or RAEB, according to the FAB classification, in combination with anemia, defined as hemoglobin level below 100 g/L or regular RBC transfusion need. Exclusion criteria were ongoing bleeding, transfusion-dependent thrombocytopenia, or eligibility for curative treatment. The poor predictive group for erythroid response, according to our validated model,9 was not eligible for the third study.2 As recently reported,16 it was possible to reclassify approximately 50% of the patients according to the WHO criteria, but the present analysis was, for practical and logistic reasons, confined to the original FAB classification. In the current long-term follow-up, 129 of the 141 patients were considered evaluable for survival. Twelve patients were nonevaluable because they did not meet the inclusion criteria (4), were excluded prior to treatment because of deterioration of concurrent disease (6), or could neither follow the treatment regimen nor be assessed for long-term outcome (2). All 129 evaluable patients were followed up per December 1, 2002, 45 months after last inclusion. A total of 123 of the 129 patients were considered evaluable for a response to treatment. The 6 patients not evaluable for response did not complete the first 6 weeks of treatment due to side effects (4), having been diagnosed with AML within 1 week (1), or concomitant severe depression (1). Baseline characteristics are shown in Table 1.
Baseline characteristics and treatment regimens of the study cohorts
Variable . | Epo-G study I . | Epo-G study II . | Epo-G study III . | Epo-G study I-III . | IPSS/IMRAW* . |
|---|---|---|---|---|---|
| No. patients evaluable for response† | 21 | 50 | 52 | 123 | NA |
| No. patients evaluable for survival‡ | 22 | 53 | 54 | 129 | 334 |
| Median age, y (range) | 69 (43-87) | 70 (48-87) | 74 (47-87) | 72 (43-87) | 70 (16-96) |
| Sex | |||||
| Male | 16 | 27 | 29 | 72 | 188 |
| Female | 6 | 26 | 25 | 57 | 146 |
| FAB | |||||
| RA | 4 | 11 | 15 | 30 | 137 |
| RARS | 6 | 13 | 22 | 41 | 73 |
| RAEB | 12 | 29 | 17 | 58 | 124 |
| IPSS§ | |||||
| Low | 6 | 11 | 17 | 34 | 78 |
| Int-1 | 9 | 21 | 27 | 57 | 169 |
| Int-2 | 4 | 14 | 5 | 23 | 66 |
| High | 0 | 1 | 3 | 4 | 21 |
| Transfusion need | |||||
| Yes | 16 | 38 | 37 | 91 | Not known |
| No | 6 | 15 | 17 | 38 | Not known |
| Predictive group for response | |||||
| Good | 9 | 17 | 17 | 43 | Not known |
| Intermediate | 6 | 20 | 19 | 45 | Not known |
| Poor | 5 | 11 | 0 | 16 | Not known |
Variable . | Epo-G study I . | Epo-G study II . | Epo-G study III . | Epo-G study I-III . | IPSS/IMRAW* . |
|---|---|---|---|---|---|
| No. patients evaluable for response† | 21 | 50 | 52 | 123 | NA |
| No. patients evaluable for survival‡ | 22 | 53 | 54 | 129 | 334 |
| Median age, y (range) | 69 (43-87) | 70 (48-87) | 74 (47-87) | 72 (43-87) | 70 (16-96) |
| Sex | |||||
| Male | 16 | 27 | 29 | 72 | 188 |
| Female | 6 | 26 | 25 | 57 | 146 |
| FAB | |||||
| RA | 4 | 11 | 15 | 30 | 137 |
| RARS | 6 | 13 | 22 | 41 | 73 |
| RAEB | 12 | 29 | 17 | 58 | 124 |
| IPSS§ | |||||
| Low | 6 | 11 | 17 | 34 | 78 |
| Int-1 | 9 | 21 | 27 | 57 | 169 |
| Int-2 | 4 | 14 | 5 | 23 | 66 |
| High | 0 | 1 | 3 | 4 | 21 |
| Transfusion need | |||||
| Yes | 16 | 38 | 37 | 91 | Not known |
| No | 6 | 15 | 17 | 38 | Not known |
| Predictive group for response | |||||
| Good | 9 | 17 | 17 | 43 | Not known |
| Intermediate | 6 | 20 | 19 | 45 | Not known |
| Poor | 5 | 11 | 0 | 16 | Not known |
NA indicates not applicable.
The IPSS/IMRAW is an untreated cohort that originates from a merger of patients from 7 different studies. Baseline data on transfusion need and S-Epo level are unknown for these patients; hence it is not possible to group them according to the predictive model for response.
Evaluability for response: evaluable for survival and completed 6 weeks of treatment.
Evaluability for survival: (1) fulfilled study criteria, (2) started treatment, (3) possible to follow up.
Eleven patients did not have a successful cytogenetic analysis and could not be scored.
The current study, as well as the 3 previous ones performed by the Nordic MDS Group, followed the guidelines of the investigation review boards of Sweden and Norway, and all patients provided writen informed consent in accordance with the Declaration of the Helsinki.
Treatment
Epo and G-CSF were given subcutaneously and, in general, patients self-administrated the drugs. The induction treatment was given in slightly different ways in the 3 studies (Table 1): G-CSF 0.3 to 3.0 μg/kg/d alone for 6 weeks and then in combination with Epo 60 to 120 U/kg/d for 12 weeks (Study I); arm A: G-CSF 30 to 150 μg/d alone for 4 weeks and then in combination with Epo 5000 to 10 000 U/d for 12 weeks; arm B: Epo alone for 8 weeks and then in combination with G-CSF for 10 weeks (same doses as in arm A) (Study II); and combination of Epo 10 000 units 5 days per week and G-CSF 75 to 300 μg 3 days per week for 12 weeks (Study III). The treatment regimens have previously been described in detail.2,6,13 In case of an erythroid response, maintenance treatment aiming at the lowest effective doses was offered in the first study and was part of the protocol in the other 2.
Response criteria
The response criteria were identical in the 3 studies. A complete erythroid response (CER) was defined as an increase in hemoglobin level to at least 115 g/L without need for RBC transfusions. A partial erythroid response (PER) required an increase in hemoglobin level of 15 g/L or more for patients with nontransfused anemia or a 100% reduction of transfusion need. These criteria have been used in the Nordic MDS Group studies2,6,13 as well as in the recently reported French randomized study,2,6,17 and they differ from the response criteria proposed by the International Working Group (IWG).18 The PER criteria of our study nearly fulfilled the criteria for IWG major erythroid response. When reclassifying our patients, 19 of 21 patients with PER had IWG major erythroid response. Only 2 of our 21 partial responders were classified as IWG minor erythroid responders. IWG minor erythroid responders who did not meet the PER criteria were not reported as responders and did not receive maintenance treatment. The date of relapse was defined as the date of first transfusion. Patients who initially achieved CER were kept in this group for the analysis of duration of response.
Comparison with the IPSS/IMRAW database
The IPSS/IMRAW study cohort consisted of 816 untreated MDS patients from 7 previous studies.5 Most patients were untreated, but short courses of treatment (less than 3 months) with either low-dose oral chemotherapy or hematopoietic growth factors were accepted. To evaluate if treatment with Epo-G affects the natural course of the disease, we made a comparison with patients selected from the IPSS/IMRAW database.
Selection of patients for the comparison
The Epo-G cohort used for the comparison included 123 of 129 patients evaluable for survival; 6 patients were excluded because they lacked data needed for the multivariate analysis. An untreated cohort was selected from the IPSS/IMRAW database to be similar to the Epo-G cohort by the following criteria: FAB groups RA/RARS/RAEB, hemoglobin (Hb) level below 100 g/L, and never treated with growth factors. The selected IPSS/IMRAW cohort consisted of 334 patients (Table 1).
Statistical analysis
Kaplan-Meier analyses were used to estimate survival, evolution of AML, and response duration from start of study in the long-term follow-up of the Epo-G cohort. Log-rank tests were used to test significance.
We compared the Epo-G cohort with the IPSS/IMRAW cohort regarding survival and time to AML evolution, from start of study and from time of diagnosis, respectively, using univariate and multivariate Cox regression analyses. In the multivariate analyses we adjusted for all major prognostic variables as fixed covariates (not time dependent). The following categories were defined on biologic basis (not data driven): age, number of cytopenias, karyotype according to the IPSS, percentage of BM blasts, and sex, according to the definitions in Tables 4 and 5.
Univariate and multivariate Cox regression analyses of survival of the Epo-G (n = 123) and the selected IPSS/IMRAW (n = 334) cohorts together
. | Total no. . | No. dead . | Unadjusted odds . | 95% CI . | P . | Adjusted odds . | 95% CI . | P . |
|---|---|---|---|---|---|---|---|---|
| Cohort | ||||||||
| IPSS/IMRAW | 334 | 217 | Ref | — | — | Ref | — | — |
| Epo-G | 123 | 99 | 0.9 | 0.7-1.2 | .56 | 0.9 | 0.7-1.2 | .55 |
| No. of cytopenias | ||||||||
| 0 or 1 | 196 | 114 | Ref | — | — | Ref | — | — |
| 2 or 3 | 261 | 202 | 2.2 | 1.7-2.7 | <.001 | 1.6 | 1.5-2.4 | <.001 |
| Karyotype* | ||||||||
| Good | 300 | 189 | Ref | — | — | Ref | — | — |
| Intermediate | 68 | 47 | 1.3 | 0.9-1.8 | .11 | 1.2 | 0.9-1.7 | .23 |
| Poor | 81 | 73 | 2.8 | 2.1-3.7 | <.001 | 2.4 | 1.8-3.2 | <.001 |
| Unknown | 8 | 7 | 1.4 | 0.7-3.0 | .38 | 0.7 | 0.3-1.6 | .45 |
| BM blasts | ||||||||
| Less than 5% | 277 | 161 | Ref | — | — | Ref | — | — |
| 5% to 10% | 121 | 105 | 2.2 | 1.8-2.9 | <.001 | 1.9 | 1.5-2.5 | <.001 |
| 11% to 20% | 59 | 50 | 3.6 | 2.6-4.9 | <.001 | 3.1 | 2.1-4.4 | <.001 |
| Age | ||||||||
| 16 to 45 y | 19 | 8 | Ref | — | — | Ref | — | — |
| 46 to 55 y | 50 | 26 | 1.0 | 0.5-2.2 | .99 | 1.4 | 0.6-3.1 | .42 |
| 56 to 65 y | 95 | 65 | 1.6 | 0.8-3.3 | .23 | 1.8 | 0.8-3.7 | .13 |
| 66 to 75 y | 133 | 92 | 1.7 | 0.8-3.6 | .14 | 2.1 | 1.0-4.4 | .04 |
| 76 to 85 y | 135 | 104 | 2.3 | 1.1-4.7 | .02 | 3.2 | 1.5-6.6 | .002 |
| More than 85 y | 25 | 21 | 3.1 | 1.4-7.1 | .01 | 3.5 | 1.5-7.9 | .003 |
| Sex | ||||||||
| Male | 255 | 190 | Ref | — | — | Ref | — | — |
| Female | 202 | 126 | 0.7 | 0.6-0.9 | .002 | 0.7 | 0.6-0.9 | .01 |
| Study | ||||||||
| Non-Epo-G study III | 405 | 280 | Ref | — | — | Ref | — | — |
| Epo-G study III | 52 | 36 | 0.8 | 0.6-1.2 | .28 | 1.0 | 0.7-1.5 | .99 |
. | Total no. . | No. dead . | Unadjusted odds . | 95% CI . | P . | Adjusted odds . | 95% CI . | P . |
|---|---|---|---|---|---|---|---|---|
| Cohort | ||||||||
| IPSS/IMRAW | 334 | 217 | Ref | — | — | Ref | — | — |
| Epo-G | 123 | 99 | 0.9 | 0.7-1.2 | .56 | 0.9 | 0.7-1.2 | .55 |
| No. of cytopenias | ||||||||
| 0 or 1 | 196 | 114 | Ref | — | — | Ref | — | — |
| 2 or 3 | 261 | 202 | 2.2 | 1.7-2.7 | <.001 | 1.6 | 1.5-2.4 | <.001 |
| Karyotype* | ||||||||
| Good | 300 | 189 | Ref | — | — | Ref | — | — |
| Intermediate | 68 | 47 | 1.3 | 0.9-1.8 | .11 | 1.2 | 0.9-1.7 | .23 |
| Poor | 81 | 73 | 2.8 | 2.1-3.7 | <.001 | 2.4 | 1.8-3.2 | <.001 |
| Unknown | 8 | 7 | 1.4 | 0.7-3.0 | .38 | 0.7 | 0.3-1.6 | .45 |
| BM blasts | ||||||||
| Less than 5% | 277 | 161 | Ref | — | — | Ref | — | — |
| 5% to 10% | 121 | 105 | 2.2 | 1.8-2.9 | <.001 | 1.9 | 1.5-2.5 | <.001 |
| 11% to 20% | 59 | 50 | 3.6 | 2.6-4.9 | <.001 | 3.1 | 2.1-4.4 | <.001 |
| Age | ||||||||
| 16 to 45 y | 19 | 8 | Ref | — | — | Ref | — | — |
| 46 to 55 y | 50 | 26 | 1.0 | 0.5-2.2 | .99 | 1.4 | 0.6-3.1 | .42 |
| 56 to 65 y | 95 | 65 | 1.6 | 0.8-3.3 | .23 | 1.8 | 0.8-3.7 | .13 |
| 66 to 75 y | 133 | 92 | 1.7 | 0.8-3.6 | .14 | 2.1 | 1.0-4.4 | .04 |
| 76 to 85 y | 135 | 104 | 2.3 | 1.1-4.7 | .02 | 3.2 | 1.5-6.6 | .002 |
| More than 85 y | 25 | 21 | 3.1 | 1.4-7.1 | .01 | 3.5 | 1.5-7.9 | .003 |
| Sex | ||||||||
| Male | 255 | 190 | Ref | — | — | Ref | — | — |
| Female | 202 | 126 | 0.7 | 0.6-0.9 | .002 | 0.7 | 0.6-0.9 | .01 |
| Study | ||||||||
| Non-Epo-G study III | 405 | 280 | Ref | — | — | Ref | — | — |
| Epo-G study III | 52 | 36 | 0.8 | 0.6-1.2 | .28 | 1.0 | 0.7-1.5 | .99 |
Ref indicates reference category.
Karyotype categories good, intermediate, and poor according to the IPSS.
Univariate and multivariate Cox regression analyses of AML of the Epo-G (n = 123) and the selected IPSS/IMRAW (n = 334) cohorts together
. | Total no. . | No. AML . | Unadjusted odds . | 95% CI . | P . | Adjusted odds . | 95% CI . | P . |
|---|---|---|---|---|---|---|---|---|
| Cohort | ||||||||
| IPSS/IMRAW | 334 | 57 | Ref | — | — | Ref | — | — |
| Epo-G | 123 | 33 | 1.3 | 0.9-2.0 | .22 | 1.3 | 0.7-2.2 | .40 |
| No. of cytopenias | ||||||||
| 0 or 1 | 196 | 26 | Ref | — | — | Ref | — | — |
| 2 or 3 | 261 | 64 | 2.8 | 1.8-4.4 | <.001 | 2.0 | 1.2-3.3 | .004 |
| Karyotype* | ||||||||
| Good | 300 | 43 | Ref | — | — | Ref | — | — |
| Intermediate | 68 | 17 | 2.1 | 1.2-3.6 | .01 | 2.0 | 1.1-3.5 | .02 |
| Poor | 81 | 27 | 4.2 | 2.6-6.9 | <.001 | 4.0 | 2.4-6.7 | <.001 |
| Unknown | 8 | 3 | 3.5 | 1.1-11.2 | .04 | 1.4 | 0.4-4.8 | .63 |
| BM blasts | ||||||||
| Less than 5% | 277 | 32 | Ref | — | — | Ref | — | — |
| 5% to 10% | 121 | 37 | 3.8 | 2.4-6.1 | <.001 | 2.8 | 1.7-4.7 | <.001 |
| 11% to 20% | 59 | 21 | 8.1 | 4.6-14.4 | <.001 | 6.3 | 3.3-12.0 | <.001 |
| Age | ||||||||
| 16 to 45 y | 19 | 5 | Ref | — | — | Ref | — | — |
| 46 to 55 y | 50 | 14 | 1.0 | 0.4-2.9 | .96 | 1.6 | 0.5-4.5 | .40 |
| 56 to 65 y | 95 | 22 | 0.9 | 0.3-2.4 | .83 | 0.9 | 0.3-2.5 | .86 |
| 66 to 75 y | 133 | 30 | 1.0 | 0.4-2.6 | .99 | 1.2 | 0.4-3.2 | .73 |
| 76 to 85 y | 135 | 17 | 0.6 | 0.2-1.6 | .32 | 0.8 | 0.3-2.2 | .66 |
| More than 85 y | 25 | 2 | 0.4 | 0.1-2.3 | .33 | 0.4 | 0.1-2.1 | .27 |
| Sex | ||||||||
| Male | 255 | 55 | Ref | — | — | Ref | — | — |
| Female | 202 | 35 | 0.7 | 0.5-1.1 | .11 | 0.8 | 0.5-1.3 | .42 |
| Study | ||||||||
| Non-Epo-G study III | 405 | 82 | Ref | — | — | Ref | — | — |
| Epo-G study III | 52 | 8 | 0.7 | 0.3-1.4 | .27 | 0.8 | 0.3-1.8 | .51 |
. | Total no. . | No. AML . | Unadjusted odds . | 95% CI . | P . | Adjusted odds . | 95% CI . | P . |
|---|---|---|---|---|---|---|---|---|
| Cohort | ||||||||
| IPSS/IMRAW | 334 | 57 | Ref | — | — | Ref | — | — |
| Epo-G | 123 | 33 | 1.3 | 0.9-2.0 | .22 | 1.3 | 0.7-2.2 | .40 |
| No. of cytopenias | ||||||||
| 0 or 1 | 196 | 26 | Ref | — | — | Ref | — | — |
| 2 or 3 | 261 | 64 | 2.8 | 1.8-4.4 | <.001 | 2.0 | 1.2-3.3 | .004 |
| Karyotype* | ||||||||
| Good | 300 | 43 | Ref | — | — | Ref | — | — |
| Intermediate | 68 | 17 | 2.1 | 1.2-3.6 | .01 | 2.0 | 1.1-3.5 | .02 |
| Poor | 81 | 27 | 4.2 | 2.6-6.9 | <.001 | 4.0 | 2.4-6.7 | <.001 |
| Unknown | 8 | 3 | 3.5 | 1.1-11.2 | .04 | 1.4 | 0.4-4.8 | .63 |
| BM blasts | ||||||||
| Less than 5% | 277 | 32 | Ref | — | — | Ref | — | — |
| 5% to 10% | 121 | 37 | 3.8 | 2.4-6.1 | <.001 | 2.8 | 1.7-4.7 | <.001 |
| 11% to 20% | 59 | 21 | 8.1 | 4.6-14.4 | <.001 | 6.3 | 3.3-12.0 | <.001 |
| Age | ||||||||
| 16 to 45 y | 19 | 5 | Ref | — | — | Ref | — | — |
| 46 to 55 y | 50 | 14 | 1.0 | 0.4-2.9 | .96 | 1.6 | 0.5-4.5 | .40 |
| 56 to 65 y | 95 | 22 | 0.9 | 0.3-2.4 | .83 | 0.9 | 0.3-2.5 | .86 |
| 66 to 75 y | 133 | 30 | 1.0 | 0.4-2.6 | .99 | 1.2 | 0.4-3.2 | .73 |
| 76 to 85 y | 135 | 17 | 0.6 | 0.2-1.6 | .32 | 0.8 | 0.3-2.2 | .66 |
| More than 85 y | 25 | 2 | 0.4 | 0.1-2.3 | .33 | 0.4 | 0.1-2.1 | .27 |
| Sex | ||||||||
| Male | 255 | 55 | Ref | — | — | Ref | — | — |
| Female | 202 | 35 | 0.7 | 0.5-1.1 | .11 | 0.8 | 0.5-1.3 | .42 |
| Study | ||||||||
| Non-Epo-G study III | 405 | 82 | Ref | — | — | Ref | — | — |
| Epo-G study III | 52 | 8 | 0.7 | 0.3-1.4 | .27 | 0.8 | 0.3-1.8 | .51 |
Ref indicates reference category.
Karyotype categories good, intermediate, and poor according to the IPSS.
The 2 study cohorts differed in one important aspect. In the IPSS/IMRAW cohort patients were followed from diagnosis, and all measures used to calculate the IPSS score were collected at time of diagnosis. In the Epo-G studies, however, the baseline patient characteristics, including IPSS scoring, as well as the starting point for calculation of survival were measured at start of study. Serial IPSS scoring has never been scientifically reported. It could be questioned whether a score calculated after a certain disease duration measures exactly the same thing as a score at time of diagnosis. The negative effects of, for example, long-standing transfusion therapy could not be disregarded in a patient who after 5 years still has the same IPSS score. To identify any possible selection effect of increasing duration of diagnosis to treatment time (DTT), we added DTT as a covariate in the multivariate analysis, comparing Epo-G patient groups with DTT 0 to 6, 6 to 12, and more than 12 months, respectively, with the IPSS/IMRAW cohort. A covariate designated Epo-G study III versus non–Epo-G study III was included to determine if the slightly different inclusion criteria in study III (only included patients belonging to the good or intermediate predictive groups for response) had an impact on outcome.
Results
Long-term follow-up of the Epo-G cohort
Better response rates in low-risk patients. Forty-eight of the 123 patients evaluable for treatment response (39%) responded; 27 (22%) had CER and 21 (17%) PER (Table 2). The response rates for RA, RARS, and RAEB, were 39%, 50%, and 31%, respectively (Table 2). The IPSS Low/Int-1 groups had a response rate of 46% versus 27% for Int-2/High (Table 2). The predictive groups good and intermediate, according to the validated model, had response rates of 60% and 18%, respectively, while only 1 of 16 patients in the poor predictive group responded to treatment (Table 2). Twenty-five of 85 transfusion-dependent patients evaluable for response (29%) became transfusion independent as a result of treatment.
Response rates and duration in subcategories of patients in the long-term follow-up of 3 studies on treatment with Epo and G-CSF
Group . | Total no. patients . | No. responding patients . | RR, % . | CER, % . | PER, % . | Median response duration, mo (range)* . | P . |
|---|---|---|---|---|---|---|---|
| All | 123 | 48 | 39 | 22 | 17 | 23 (3-116) | — |
| Complete responders | 27 | NA | NA | NA | NA | 29 (4-116) | .006 |
| Partial responders | 21 | NA | NA | NA | NA | 12 (3-93) | — |
| FAB | |||||||
| RA | 28 | 11 | 39 | 18 | 21 | 27 (4-62) | .83† |
| RARS | 40 | 20 | 50 | 38 | 13 | 28 (3-116) | — |
| RA + RARS | 68 | 31 | 46 | 29 | 16 | 28 (3-116) | .04‡ |
| RAEB | 55 | 17 | 31 | 13 | 18 | 11 (3-93) | — |
| IPSS | |||||||
| Low/Int-1 | 88 | 40 | 46 | 27 | 18 | 25 (3-116) | .002 |
| Int-2/High | 26 | 7 | 27 | 12 | 15 | 7 (3-23) | — |
| Predictive group for response | |||||||
| Good | 42 | 25 | 60 | 36 | 24 | 24 (3-116) | .95§ |
| Intermediate | 44 | 8 | 18 | 9 | 9 | 23 (4-93) | — |
| Poor | 16 | 1 | 6 | 0 | 6 | 3∥ | — |
Group . | Total no. patients . | No. responding patients . | RR, % . | CER, % . | PER, % . | Median response duration, mo (range)* . | P . |
|---|---|---|---|---|---|---|---|
| All | 123 | 48 | 39 | 22 | 17 | 23 (3-116) | — |
| Complete responders | 27 | NA | NA | NA | NA | 29 (4-116) | .006 |
| Partial responders | 21 | NA | NA | NA | NA | 12 (3-93) | — |
| FAB | |||||||
| RA | 28 | 11 | 39 | 18 | 21 | 27 (4-62) | .83† |
| RARS | 40 | 20 | 50 | 38 | 13 | 28 (3-116) | — |
| RA + RARS | 68 | 31 | 46 | 29 | 16 | 28 (3-116) | .04‡ |
| RAEB | 55 | 17 | 31 | 13 | 18 | 11 (3-93) | — |
| IPSS | |||||||
| Low/Int-1 | 88 | 40 | 46 | 27 | 18 | 25 (3-116) | .002 |
| Int-2/High | 26 | 7 | 27 | 12 | 15 | 7 (3-23) | — |
| Predictive group for response | |||||||
| Good | 42 | 25 | 60 | 36 | 24 | 24 (3-116) | .95§ |
| Intermediate | 44 | 8 | 18 | 9 | 9 | 23 (4-93) | — |
| Poor | 16 | 1 | 6 | 0 | 6 | 3∥ | — |
RR indicates response rate; NA, not applicable.
Only for the 41 patients entering the maintenance phase.
P value of RA versus RARS.
P value of RA + RARS versus RAEB.
P value of predictive group good versus intermediate.
There is no range because this figure represents one patient.
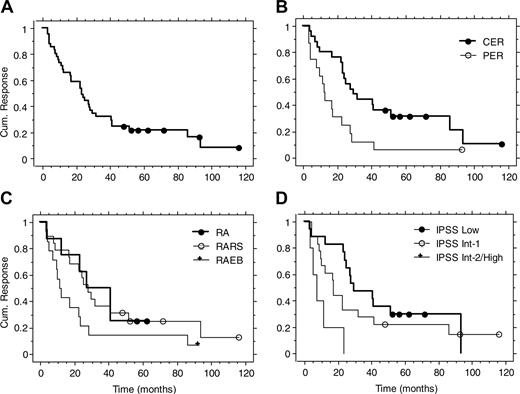
Durable responses in subsets of patients. Maintenance treatment was an option in the first study and part of the protocol in the other 2. Forty-one of the 48 responding patients were evaluable for response duration. Seven patients were considered nonevaluable because they did not enter the maintenance phase. The reasons for discontinuing treatment were patient's choice or severe concomitant disease.2,6 The overall median response duration was 23 months (range, 3-116 months or more; Table 2 and Figure 1). There was no difference between RA and RARS (P = .83; Table 2 and Figure 1). Response duration was more durable in RA plus RARS than in RAEB (median, 28 versus 11 months, P = .04; Table 2). IPSS Low/Int-1 showed longer response duration compared with Int-2/High (median, 25 versus 7 months, P = .002; Table 2). Patients with CER had longer response duration than patients with PER (median, 29 versus 12 months, P = .006; Table 2 and Figure 1). The response durations for the good and intermediate predictive groups for erythroid response were similar (median, 24 and 23 months, respectively, P = .95; Table 2). The single responding patient in the poor predictive group had a response duration of only 3 months. Overall, 20% of responses were maintained more than 4 years.
Response duration in the long-term follow-up of 3 previous studies on treatment with Epo and G-CSF. Kaplan-Meier curves for all patients (A), CER versus PER (B), FAB groups (C), and IPSS groups (D).
Response duration in the long-term follow-up of 3 previous studies on treatment with Epo and G-CSF. Kaplan-Meier curves for all patients (A), CER versus PER (B), FAB groups (C), and IPSS groups (D).
Loss of response often without signs of transformation. All patients in the study were evaluated with a BM examination after treatment was stopped, with the exception of 4 patients with severe concomitant disease. Nine of the 48 responders were still in response at time of follow-up. The reasons for relapse of anemia or discontinuation of treatment in the remaining 39 responders were loss of response without evidence of significant increase of marrow blasts (18 patients), AML transformation or significant increase of marrow blasts (7 patients), patient's decision to stop but no evidence of disease progression (6 patients), severe concurrent disease or palliative treatment (4 patients), side effects but no evidence of disease progression (2 patients), and unknown (2 patients). No patient in the study who received Epo-G developed pure red cell aplasia.
Low doses of growth factors often sufficient. We identified the lowest doses required to maintain a stable response in a review of all 18 responding patients from the third study and 6 from the second study. For RA (7 patients), RARS (11 patients), and RAEB (6 patients), the median minimal doses of Epo were 30 000 U/wk (range, 5000-50 000 U/wk), 30 000 U/wk (range, 10 000-70 000 U/wk), and 40 000 U/wk (range, 15 000-50 000 U/wk), respectively. The median minimal doses of G-CSF for RA, RARS, and RAEB were 225 μg/wk (range, 0-525 μg/wk), 90 μg/wk (range, 0-900 μg/wk), and 375 μg/wk (range, 225-450 μg/wk), respectively. These differences between FAB groups were not significant. In total, 3 of these patients were able to stop G-CSF completely and still maintain a response (2 RA, 1 RARS), and 6 patients required Epo 15 000 U/wk or lower (3 RA, 2 RARS, 1 RAEB).
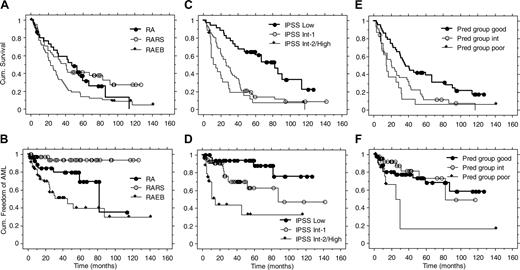
Better survival for patients in good predictive group for response and patients in the IPSS low-risk category. All 129 patients were followed up regarding survival. The overall median survival time from start of study was 31 months (range, 2-142 months or more). There was no difference between RA and RARS (P = .56; Figure 2). RA plus RARS had a longer median survival compared with RAEB (42 versus 23 months, P = .004). IPSS Low patients had a longer median survival compared with Int-1 patients (83 versus 26 months, P < .001), while the difference between Int-1 versus Int-2/High patients was not significant (26 versus 14 months, P = .06; Figure 2). There was a significant difference in survival between patients in the good and intermediate predictive groups for erythroid response (37 versus 21 months, P = .005; Figure 2). There was no difference between patients in the intermediate and poor predictive groups (21 versus 15 months, P = .40; Figure 2).
RARS patients and long-term responders rarely develop AML. A total of 124 of the 129 patients were eligible for follow-up regarding AML evolution from start of study. Five patients were excluded because their AML status was unknown at time of death or last follow-up. The cumulative incidence of AML at 4 years from start of study was 30% (Table 3).
Evolution of AML in the long-term follow-up of 3 previous studies on treatment with Epo and G-CSF
Category . | Total no. . | AML, no. (%) . | Median observation time, mo* . | Cumulative incidence of AML at 4 y, % . |
|---|---|---|---|---|
| Total | 124 | 33 (27) | 24 | 30 |
| FAB | ||||
| RA | 28 | 7 (25) | 43 | 21 |
| RARS | 40 | 2 (5) | 32 | 6 |
| RAEB | 56 | 24 (43) | 17 | 55 |
| IPSS | ||||
| Low | 33 | 4 (12) | 59 | 6 |
| Int-1 | 56 | 14 (25) | 24 | 30 |
| Int-2/High | 26 | 12 (46) | 8 | 67 |
| Predictive group | ||||
| Good | 42 | 12 (29) | 34 | 27 |
| Intermediate | 44 | 7 (16) | 20 | 18 |
| Poor | 16 | 7 (44) | 11 | 83 |
Category . | Total no. . | AML, no. (%) . | Median observation time, mo* . | Cumulative incidence of AML at 4 y, % . |
|---|---|---|---|---|
| Total | 124 | 33 (27) | 24 | 30 |
| FAB | ||||
| RA | 28 | 7 (25) | 43 | 21 |
| RARS | 40 | 2 (5) | 32 | 6 |
| RAEB | 56 | 24 (43) | 17 | 55 |
| IPSS | ||||
| Low | 33 | 4 (12) | 59 | 6 |
| Int-1 | 56 | 14 (25) | 24 | 30 |
| Int-2/High | 26 | 12 (46) | 8 | 67 |
| Predictive group | ||||
| Good | 42 | 12 (29) | 34 | 27 |
| Intermediate | 44 | 7 (16) | 20 | 18 |
| Poor | 16 | 7 (44) | 11 | 83 |
Median observation time from start of study, censored at time of AML.
Only 2 of 40 RARS patients developed AML during a median observation time of 32 months (observation time censored at time of AML; Table 3). Both patients had IPSS Int-1, one with complete and one with no response. By using Kaplan-Meier estimation, as shown in Figure 2, the time until 25% of patients developed AML was significantly longer for RA compared with RAEB (60 versus 13 months, P = .04), for IPSS Low compared with Int-1 (more than 127 versus 30 months, P = .02), and for IPSS Int-1 compared with Int-2/High (30 versus 6 months, P = .003). The time until 25% of patients developed AML in the good and intermediate predictive groups for erythroid response was significantly longer than for the poor predictive group (52 versus 13 months, P = .008). Only 1 of 20 patients with a response lasting more than 2 years developed AML.
Survival and AML evolution in the long-term follow-up of 3 previous studies on treatment with Epo and G-CSF. Kaplan-Meier curves for FAB groups (A-B), IPSS groups (C-D), and predictive groups for response (E-F).
Survival and AML evolution in the long-term follow-up of 3 previous studies on treatment with Epo and G-CSF. Kaplan-Meier curves for FAB groups (A-B), IPSS groups (C-D), and predictive groups for response (E-F).
Comparison of Epo-G and IPSS/IMRAW cohorts
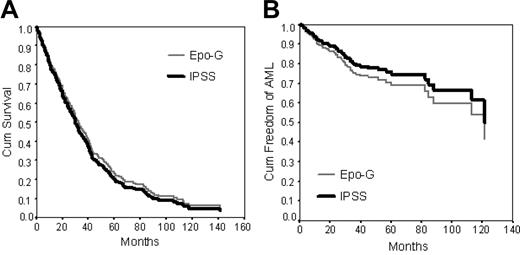
The survival of the Epo-G cohort does not differ from the IPSS/IMRAW cohort. In the multivariate analysis, all major prognostic variables (percentage of BM blasts, karyotype, and number of cytopenias) as well as age had significant impact on survival. There were no significant differences in survival between the Epo-G and the IPSS/IMRAW cohorts (P = .55; Table 4 and Figure 3A).
Because survival was measured from time of diagnosis in the IPSS/IMRAW cohort and from start of study in the Epo-G cohort, we assessed the effect of increasing DTT in the Epo-G cohort by adding DTT as a covariate (Epo-G DTT 0 to 6, 6 to 12, and more than 12 months versus IPSS). No significant effects on survival were seen with increasing DTT in the Epo-G cohort (data not shown).
Patient groups with BM blast counts above or below 5% were analyzed separately with multivariate analysis. In the group with BM blasts below 5%, there was no difference in survival of treated compared with untreated patients, the odds ratio (OR) being 1.3 (95% confidence interval [CI], 0.9-2.1; P = .18). However, there was a difference in survival in the group with BM blasts above or equal to 5%, where treated patients survived longer compared with untreated patients (OR, 0.6; 95% CI, 0.4-1.0; P = .04).
In the analysis of all patients (Epo-G and IPSS/IMRAW cohorts), female sex was associated with better survival compared with male sex, with an OR of 0.7 (95% CI, 0.6-0.9; P = .01). To investigate whether treatment with Epo and G-CSF affected survival for men or women differently, we compared each sex separately. Epo-G–treated male patients had a somewhat lower OR of 0.7 when compared with the IPSS/IMRAW males (95% CI, 0.5-1.0; P = .07). For women there was no difference in survival between the treated and untreated patients (OR, 1.3; 95% CI, 0.8-2.0; P = .32).
Epo-G does not increase the overall risk of AML. All major prognostic variables (percentage of BM blasts, karyotype, and number of cytopenias) had significant impact on AML evolution in the multivariate analysis. Risk of transformation to AML did not vary with age. There was no difference in risk of AML evolution between the Epo-G and the IPSS cohorts (P = .40; Table 5 and Figure 3B).
As for survival, we assessed the possible impact of selection for longer DTT in the Epo-G cohort by adding DTT as a covariate. There was no consistent pattern; the Epo-G patient groups showed the following ORs in comparison with the IPSS/IMRAW cohort: DTT 0 to 6 months (OR, 2.1; P = .02), DTT 6 to 12 months (OR, 0.3; P = .09), and DTT more than 12 months (OR, 1.0; P = .96).
As for survival, we analyzed the risk of AML evolution in patient groups with BM blast counts above or below 5% separately. For patients with BM blasts below 5%, there was no difference in treated compared with untreated patients (OR, 1.3; 95% CI, 0.5-3.5; P = .66). Neither was there a difference in treated compared with untreated patients in the group with BM blasts above or equal to 5% (OR, 1.3; 95% CI, 0.7-2.4; P = .49).
There was no overall difference in the risk of AML transformation between men and women (P = .42; Table 5). Furthermore, there was no difference between the Epo-G–treated and the IPSS/IMRAW men (OR, 0.7; 95% CI, 0.3-1.6; P = .41). However, women in the Epo-G cohort had an increased OR of 3.6 (95% CI, 1.5-8.2; P = .003) when compared with the IPSS/IMRAW women. This could not be explained by differences in risk score or age because these variables were used as covariates and adjusted for in the multivariate analysis. We added 5q- as sole cytogenetic aberration as a covariate, but the result remained unchanged. We then compared males with females within the Epo-G and IPSS/IMRAW cohorts separately. In the Epo-G cohort, women had higher odds compared with men, but the difference was not significant (OR, 1.9; 95% CI, 0.9-4.1; P = .11). However, in the selected IPSS/IMRAW cohort, women developed significantly less AML than men (OR, 0.5; 95% CI, 0.3-0.9; P = .03). The proportions of high-risk patients (IPSS Int-2/High) were well balanced between the sexes in the selected IPSS/IMRAW cohort (19.1 and 18.8%, respectively), and the risk variables included in the IPSS score were adjusted for in the analysis. The sex difference regarding the risk of AML evolution was only seen in the selected IPSS/IMRAW study cohort and not in the unselected IPSS/IMRAW dataset.
Multivariate Cox regression curves of the Epo-G compared with the IPSS/IMRAW cohort. Survival (A) and AML evolution (B).
Multivariate Cox regression curves of the Epo-G compared with the IPSS/IMRAW cohort. Survival (A) and AML evolution (B).
Discussion
We present a long-term follow-up study of a large cohort of MDS patients treated with Epo-G with a nearly complete recording of all pivotal data and with 45 months between last inclusion and time of follow-up. The results allow us to make conclusions about long-term outcome of treatment regarding response duration, survival, and AML evolution and to present treatment recommendations.
Epo, with or without the addition of G-CSF, has been promoted as part of the standard treatment for low-risk MDS in several guideline publications,19-21 albeit no country has formal approval of the treatment. In the present study, the overall response rate was 39% and the median response duration was 23 months, which is longer than what has been reported for Epo alone.22 As many as 29% of transfusion-dependent patients became transfusion independent. Patients in the good predictive group for erythroid response showed a response rate of 60% and a median response duration of 24 months. The median response duration for patients in the intermediate predictive group was similar—23 months—despite the lower total response rate of 18%. In contrast, only 1 of 16 patients (6%) in the poor predictive group showed a response, which lasted for 3 months. These results support the usefulness of the validated predictive model also for prediction of response duration. Not surprisingly, IPSS Low/Int-1 patients responded better than Int-2/High, with a response rate of 46% versus 27% and median response duration of 25 versus 7 months. Based on these results, we can conclude that neither patients with poor probability of response according to the model nor those with IPSS scores Int-2 or High are suitable candidates for treatment with Epo-G.
We also analyzed the reasons for relapse of anemia. Most patients who lost their response appeared to escape the effect of growth factor treatment without signs of pure red cell aplasia, progression of marrow blasts, or overt transformation to AML. In general, these patients did not respond to increased doses of growth factors. Only 7 of 48 responders developed signs of transformation or disease progression in terms of increased BM blasts during treatment.
The induction treatment regimen used in the last study2 started with Epo 60 000 U/wk divided in 3 doses, with the addition of G-CSF 300 μg/wk after 8 weeks in case of no response. In case of a CER, the Epo dose was gradually reduced. The G-CSF dose was adjusted aiming at a neutrophil count of 6 × 109/L to 10 × 109/L. This is a practical model for treatment, but it does not exclude other efficacious modalities. A recently published pilot study showed that once-weekly dosing of Epo is probably feasible,23 but larger studies are needed to confirm this finding. In the present study, required maintenance doses of Epo and G-CSF did not differ significantly between the FAB groups. The median Epo dose was 30 000 U/wk—thus around half of the induction dose. In general, low G-CSF doses were required during maintenance treatment (median, 225 μg/wk; range, 0 -900 μg/wk). The effective doses of G-CSF seem to be significantly lower than those required for treatment of neutropenia or stem cell mobilization.24 Several studies have independently demonstrated a synergistic effect between Epo and G-CSF on the anemia of MDS.6-8 Interestingly, we showed that patients with RARS, in which G-CSF has been shown to have the strongest synergistic effect on erythropoiesis, required very low G-CSF doses, with a median of only 90 μg/wk.
Patients were included in the long-term follow-up analysis for survival and AML as soon as they had achieved 1 day of treatment. We observed, as expected, a better survival in the low-risk versus the high-risk categories. We also observed a significantly better survival for patients in the good predictive group for erythroid response than for those in the intermediate and poor predictive groups. This is interesting because the predictive model is based on S-Epo and degree of transfusion need, which are variables not usually considered to predict survival. However, a correlation between S-Epo and survival has previously been reported in a smaller study.25
As expected, patients with RARS and IPSS-Low showed the lowest cumulative incidences of AML: 6% for both categories at 4 years from start of treatment. More unexpected was the finding that the predictive model for response also predicted for transformation to AML. Patients in the good and intermediate predictive groups developed less AML than patients in the poor predictive group: 27% and 18% versus 83% after 4 years, respectively. The superior survival and lower risk of AML evolution seen in the good compared with the poor predictive group for response is partly explained by covariations in risk factors. It is nevertheless important because it further supports the use of proerythroid growth factors in the good and intermediate predictive groups and should discourage it in the poor predictive group.
One of 5 responses lasted longer than 4 years, a few even 10 years. Only 1 of 20 patients responding over 2 years developed AML. Hence, Epo and G-CSF keeps a proportion of patients free of transfusions for several years without apparent increased risk of AML evolution.
It was not possible to reclassify every patient in the Epo-G cohort according to the WHO criteria.4 However, we recently reported follow-up data of 64 patients originating from the present treated cohort who were reclassified according to the WHO criteria.16 Interestingly, we showed that patients with RARS had significantly higher erythroid response rate than patients with refractory cytopenia with multilineage dysplasia and ringed sideroblasts (RCMD/RS). This difference correlated well with the predictive model for erythroid response, because most RARS patients belonged to the good predictive group, while most RCMD/RS patients belonged to the intermediate predictive group. Furthermore, RA plus RARS had significantly longer median survival compared with RCMD plus RCMD/RS.
To further investigate the influence of Epo-G treatment on long-term outcome, we compared our cohort of treated patients with an untreated cohort selected from the IPSS/IMRAW database. There was no evidence that treatment with Epo-G influenced survival when compared with the untreated cohort. We observed, as expected, that all major prognostic variables (BM blasts, karyotype, and number of cytopenias) as well as age had significant impact on survival. Also, women survived longer than men. We conducted 2 subgroup analyses of the combined Epo-G and IPSS/IMRAW cohort, comparing treated with untreated patients within groups with high or low percentage of BM blasts as well as within each sex separately. In the subgroup of patients with BM blasts above or equal to 5%, treated patients had significantly better survival compared with untreated patients, while there was no difference in the risk for AML evolution. Whether or not this is a chance finding remains to be investigated further. There was no difference in survival between treated and untreated patients in the cohort with BM blasts below 5% or within each sex separately.
There was no difference in AML evolution between treated and untreated patients when analyzing the Epo-G and IPSS/IMRAW cohorts together. As expected, we saw that all major prognostic variables (BM blasts, karyotype, and number of cytopenias) had significant impact on the risk of AML, while sex was not a risk factor for AML. We conducted subgroup analyses focusing on groups with high or low percentage of BM blasts as well as each sex separately. There was no difference in risk of AML evolution between treated and untreated patients within subgroups with high or low percentage of BM blasts, respectively. When analyzing males separately, there was no difference between treated and untreated patients. However, when focusing only on females, treated females developed more AML than untreated ones. We undertook further analyses to account for this unexpected finding. Within the Epo-G cohort alone, women developed more AML than men, but the difference was not significant. In the overall, nonselected, IPSS/IMRAW material, female sex was not a risk factor for AML.5 However, in our study cohort of untreated anemic patients with RA, RARS, or RAEB, selected from the IPSS/IMRAW database, females develop significantly less AML than males. This is not explained by imbalance of the major risk variables or the frequency of patients with 5q- as sole cytogenetic aberration, because adjustment failed to eliminate the difference. Because a difference in risk of AML by sex was not an a priori hypothesis, this could be a chance finding.
We measured survival and time to AML evolution from start of study in the Epo-G cohort and from time of diagnosis in the IPSS/IMRAW cohort. In the Epo-G cohort, the IPSS score was measured at start of study. The median DTT in the Epo-G cohort was 6 months. To investigate if increasing DTT had an impact on outcome, we added DTT as a covariate in the multivariate analysis. We found no difference in survival in the Epo-G strata with different DTT, compared with the IPSS/IMRAW cohort. Neither was there a consistent difference in risk of AML, although we observed a difference between the groups with DTT 0 to 6 (OR, 2.1) and 6 to 12 (OR, 0.3) months, which deserves further consideration. A possible clinical interpretation is that growth factor treatment should be confined to patients in whom a stable percentage of BM blasts has been documented in 2 consecutive BM investigations.
We conclude that Epo and G-CSF treatment does not affect overall survival or risk of AML evolution in comparison with supportive care only. Patients likely to benefit from treatment with Epo and G-CSF have high or intermediate probability of erythroid response according to the predictive model and belong to the IPSS categories Low or Int-1. Moreover, a certain documentation of disease stability is warranted. These patient categories are expected to have high response rates, long response durations, and no negative effect on survival or risk of AML evolution.
Prepublished online as Blood First Edition Paper, April 19, 2005; DOI 10.1182/blood-2004-10-3872.
Supported by grants from the Swedish Society of Cancer (3689-B03-09XAA), The Cancer Society of Stockholm (041131), and the Nordic Cancer Union (4796-B03-01XAAN).
M.J. performed the follow-up study, interpreted the data, made the statistical calculations with supervision from S.M.M. and E.H.-L., and wrote the manuscript. S.M.M. was responsible for the statistics. I.D. was responsible for follow-up of the Norwegian patients. A.P.-M. was responsible for the bone marrow morphology. E.H.-L. planned and performed the 3 original Epo-G studies and supervised the follow-up study and the interpretation of data.
Presented orally in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 8, 2003.1
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Drs Peter Greenberg and Christopher Cox of the International MDS Risk Analysis Workshop for their invaluable comments on the manuscript and for providing access to the IPSS/IMRAW database. We also thank Drs Jan Astermark, Magnus Carlsson, Inger-Marie Dahl, Lena-Maria Engström, Gunnar Grimfors, Eva Hesse-Sundin, Martin Hjorth, Gunnar Juliusson, Olle Linder, Michaela Luthman, Herman Nilsson-Ehle, Lars Nilsson, Gunnar Öberg, Jan Samuelsson, Jon Magnus Tangen, and Ingemar Winqvist of the Nordic MDS Group for valuable help with the long-term follow-up part of the study (International MDS Risk Analysis Workshop).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal