Comment on Cooperman et al, page 1084
In humans, iron-limited erythropoiesis can be caused by total body lack of iron, decreased iron exit from macrophages, or absence of transferrin, the molecule that presents iron for receptor-mediated uptake by erythroid precursors. Cooperman and colleagues report that deficiency of iron regulatory protein 2 in mice leads to iron-limited erythropoiesis because of reduced transferrin receptor expression in erythroid precursors.
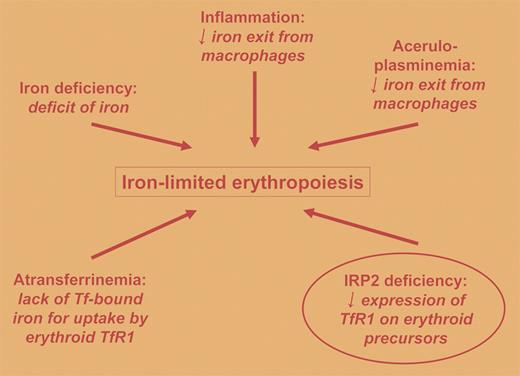
Under normal circumstances, most of the body's iron is present in the hemoglobin of erythrocytes1 and, quantitatively speaking, most of the transferrin receptors in the body are expressed on erythroid precursors2 for the purpose of acquiring iron for these hemoglobin-synthesizing factories. Impairment of iron acquisition by erythroid precursors is the proximal cause of iron deficiency anemia and the anemia of congenital atransferrinemia, and it likely contributes to the anemia of chronic inflammation and the anemia of congenital aceruloplasminemia (4 conditions of iron-limited erythropoiesis). Iron regulatory protein (IRP) 1 and 2 are central to the posttranscriptional regulation of transferrin receptor 1 (TfR1) expression,3 with decreased IRP levels leading to lower amounts of membrane TfR1, but heretofore IRP deficiency has not been identified as a cause of anemia of iron-limited erythropoiesis.FIG1
Causes and mechanisms of iron-limited erythropoiesis are summarized. The human conditions are not circled. The contribution to iron-limited erythropoiesis of IRP2 deficiency in mice is circled. Tf indicates transferrin;TfR1, transferrin receptor 1; and IRP2, iron regulatory protein 2.
Causes and mechanisms of iron-limited erythropoiesis are summarized. The human conditions are not circled. The contribution to iron-limited erythropoiesis of IRP2 deficiency in mice is circled. Tf indicates transferrin;TfR1, transferrin receptor 1; and IRP2, iron regulatory protein 2.
Cooperman and colleagues have carefully investigated erythropoiesis in mice with targeted deletion of IRP2, which previously has been shown to lead to a neurodegenerative condition. They report that IRP2-deficient mice have reduced expression of TfR1 in erythroid precursors and, in keeping with other conditions of iron-limited erythropoiesis, these mice develop microcytic anemia (see the figure). In contrast to iron deficiency anemia and the anemia of chronic inflammation, the anemia of IRP2 deficiency is associated with normal to increased transferrin saturation. The constellation of microcytic anemia, increased tissue iron deposition, and neurodegeneration in IRP2-deficient mice parallels the clinical findings of congenital aceruloplasminemia,4 while the constellation of microcytic anemia, reduced macrophage iron, and increased hepatic iron parallels the manifestations of congenital atransferrinemia.5 The lesser degree of tissue iron-loading seen with IRP2 deficiency versus congenital atransferrinemia is consistent with the observation that IRP2 deficiency does not completely abolish TfR1 expression on erythroid precursors, whereas the nearly complete absence of transferrin would effectively abolish iron uptake via TfR1.
In searching for human correlates of IRP2 deficiency in mice, one must keep in mind not only microcyic anemia, tissue iron-loading, and neurogeneration, but also the observation by Cooperman and colleagues that these mice have elevated free erythrocyte protoporphyrin levels in erythrocytes, in the range seen with erythropoietic protoporphyria. These markedly elevated protoporphyrin levels are probably due to the combination of inadequate iron in the erythropoietic precursors plus marked posttranslational up-regulation of delta aminolevulinc acid synthase associated with IRP2 deficiency. Cutaneous manifestations were not reported in the IRP2-deficient mice. In view of the fascinating picture of murine IRP2 deficiency presented by the present report and previous publications from these investigators, one senses that the involvement of IRP2 with a spectrum of human diseases is a fascinating book yet to be written.
This work was supported in part by NIH research grant No. UH1-HL03679-05 from the National Heart, Lung and Blood Institute and the Office of Research on Minority Health and by Howard University General Clinical Research Center Grant No. MO1-RR10284. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal