Comment on Beilhack et al, page 1113
A new imaging technique reveals T cells' journey into GVHD target organs.
Graft-versus-host disease (GVHD) remains the major toxicity of allogeneic bone marrow transplantation (BMT) and prevents the broader use of this potentially curative therapy.1 The liver, skin and gastrointestinal (GI) tract are primarily affected by acute GVHD, although the lung is also probably a target.2 This distribution of organs is unusual, because kidney and heart allografts are also subject to immunologic attack (ie, rejection), yet they are not targets of acute GVHD. The elegant study of Beilhack and colleagues from Stanford in this issue ofBlood takes us one step closer to understanding this unusual clustering.
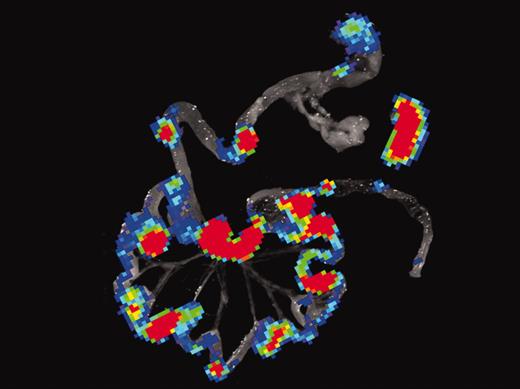
To track the movements of cells in vivo, the Stanford team used a technique of bioluminescence imaging, similar to PET scanning. They first engineered the T cells to glow in the dark, and then tracked their movements in a serial fashion after allogeneic BMT. Within 12 hours, the T cells homed to secondary lymphoid organs in the abdomen. Within 96 hours, donor T cells had divided at least half a dozen times (a 64-fold increase) and could be easily detected throughout the GI tract (see figure). No such proliferation occurred in recipients of syngeneic BMT that did not develop GVHD. Additional experiments documented increased expression of gut homing receptors on proliferating T cells. In the absence of homing receptors, T cells did not cause GVHD when transplanted, even though they proliferated in vitro to antigen presenting cells of the host.FIG1
Bioluminescence imaging (BLI) of gastrointestinal tissues and spleen combined with histology and immunofluorescence microscopy of Peyer patches during induction of acute GVHD. See the complete figure in the article beginning on page 1113.
Bioluminescence imaging (BLI) of gastrointestinal tissues and spleen combined with histology and immunofluorescence microscopy of Peyer patches during induction of acute GVHD. See the complete figure in the article beginning on page 1113.
These findings provide a fascinating glimpse of early T-cell behavior during GVHD, and they underscore several important points. First, the gut-associated lymphoid tissue and the spleen are the first destinations for T cells in an allogeneic host, and they find their targets rapidly. Other groups have also shown this early migration to the GI tract during GVHD, which helps explain the primacy of the GI tract as a GVHD target organ.3,4 Indeed, the mucosal surface of the GI tract is largely destroyed within 1 week after transplantation in many mouse BMT models, undoubtedly contributing to the rapid mortality. GVHD prophylaxis strategies that specifically target the GI tract may therefore have broad systemic effects and help reduce transplantation-related mortality.
Second, the speed of T-cell activation and proliferation after infusion reminds us that the T cells causing GVHD are already mature and do not need to differentiate from stem cells present in the donor inoculum. Within hours, T cells migrate out of the bloodstream into intestinal and splenic lymphoid tissue, where they proliferate. This rapid transit time is particularly pertinent in clinical medicine where therapeutic levels of calcineurin inhibitors are often not achieved until several days after stem cell infusion. Subtherapeutic levels at a time of such intense T-cell stimulation may have dramatic consequences on eventual GVHD severity, even though the clinical symptoms may not be apparent for another 2 weeks.
Third, the immediate homing of T cells to lymphoid organs reinforces the fact that the primary target organ of the GVHD is the lymphohematopoietic stem of the recipient.5 Ablation of host blood–derived components by the donor immune system is usually not appreciated because donor stem cells engraft and eventually support peripheral blood counts. In addition, high-dose chemotherapy ablates such a large percentage of host marrow and lymphoid elements that we often do not recognize the ablative power of the donor immune system. Such ablation is, however, a clinically prominent feature of transfusion-associated GVHD, where marrow aplasia is often a proximal cause of death.
The increased expression of homing receptors for the GI tract on T cells shortly after BMT emphasizes the importance of these receptors, and the fact that T cells lacking homing receptors did not induce GVHD in vivo even though they responded vigorously to alloantigens in vitro. This discrepancy may be a partial explanation as to why the mixed leukocyte reaction does not predict the incidence or severity of GVHD; if T cells can't traffic to sites of stimulation, they won't be stimulated. It also suggests hope for novel GVHD therapeutic strategies that paralyze T cells rather than simply choking them to death. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal