Comment on Boggon et al, page 996
Jaks, in general, are critical for cytokine signaling and Jak3 is crucial for key immunoregulatory cytokines. The study by Boggon and colleagues provides the first insights into the structure of these key tyrosine kinases.
Cytokines, key regulators of cell development and homeostasis, are especially important for hematopoiesis and immunoregulation. A subset of cytokines bind receptors denoted as Type I and II cytokine receptors, and these receptors associate with a family of cytoplasmic protein tyrosine kinases known as Janus kinases, or Jaks.1,2 While we know from various genetic approaches that Jaks have distinct but essential functions in initiating cytokine signaling, a precise understanding of their regulation has been hampered by the absence of 3-dimensional structural information. In this issue of Blood, Boggon and colleagues begin to fill this gap by providing the first glimpse of the structure of a Jak kinase domain. This work reveals interesting structural similarities and differences with other protein tyrosine kinases. Equally important, this new information has the potential for optimizing the selectivity of Jak antagonists.
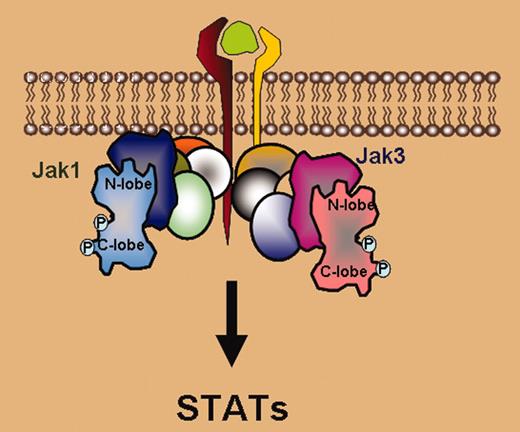
There are 4 members of the Janus kinase family: Jak1, Jak2, Jak3, and Tyk2.3 Each has distinct functions based on its ability to bind different cytokine receptors. The present study focuses on Jak3, which specifically associates with the common gamma chain γc.4 This is a shared receptor subunit for interleukin (IL)–2, IL-4, IL-7, IL-9, IL-15, and IL-21. These cytokines are critical for immunoregulation, with IL-7 being especially crucial for lymphoid development. Importantly, mutations of the IL-7 receptor, γc, or Jak3 underlie the majority of cases of severe combined immunodeficiency (SCID).5 This discovery led to the idea that Jak3 inhibitors might represent a new class of immunosuppressant drugs. In fact, a potent, selective, orally available Jak3 inhibitor has now been generated.5 This drug is efficacious in preclinical models of transplant rejection.6 FIG1
Janus kinases, multidomain proteins consisting of a catalytic or kinase domain associated with pseudokinase, SH2, and FERM domains, associate with cytokine receptors. Upon ligand binding, Janus kinases are activated resulting in their phosphorylation. According to Boggon et al, the Jak3 kinase domain has a typical bilobed structure. Phosphorylation of tyrosine 981 in the activation loop allows interaction with arginine residues in the C-helix of the N lobe, allowing the opening of the binding site cleft. Activation of the kinase results in phosphorylation of the receptor and subsequent activation of the cytosolic family of transcription factor signal transducers and activators of transcription (STATs). Boggon et al also identified other unique structural features of the Jak3 kinase domain, which may mediate interactions of the kinase domain with other regulatory domains.
Janus kinases, multidomain proteins consisting of a catalytic or kinase domain associated with pseudokinase, SH2, and FERM domains, associate with cytokine receptors. Upon ligand binding, Janus kinases are activated resulting in their phosphorylation. According to Boggon et al, the Jak3 kinase domain has a typical bilobed structure. Phosphorylation of tyrosine 981 in the activation loop allows interaction with arginine residues in the C-helix of the N lobe, allowing the opening of the binding site cleft. Activation of the kinase results in phosphorylation of the receptor and subsequent activation of the cytosolic family of transcription factor signal transducers and activators of transcription (STATs). Boggon et al also identified other unique structural features of the Jak3 kinase domain, which may mediate interactions of the kinase domain with other regulatory domains.
The new study reports the successful solution of the crystal structure of the Jak3 kinase domain with a nonselective kinase inhibitor. Not surprisingly, the work reveals that this domain has the classic bilobed (N- and C-lobe) composition seen in all other protein kinases. Furthermore, the overall conformation is similar to well-studied tyrosine kinases such as lymphocyte-specific kinase (Lck), the insulin receptor kinase, and Ableson kinase (Abl). However, it appears that the active state of the kinase is directly coupled to the tyrosine-phosphorylated activation loop. In addition to the kinase domain, Jaks comprise band 4.1, ezrin, radixin, moesin (FERM)–, src homology 2 (SH2)–, and pseudokinase domains; the latter domain is unique to Jaks. Boggon et al noted additional unique structural features of the Jak3 kinase domain, which they suggest may mediate interdomain contacts. They identified an additional helix in the C-lobe, referred to as the αFG helix. They also noted that the structure of the loop between β2 and β3 is distinct and creates a pocket in the posterior on the N-lobe. The authors predict, based on primary structure, that these features may be conserved in other vertebrate Jaks. This is of interest, as we know from several lines of evidence that pseudokinase and FERM domains have essential regulatory functions with respect to catalytic activity. Mutations in the Jak3 pseudokinase domain result in SCID, whereas mutations in the Jak2 pseudokinase domain have recently been shown to underlie polycythemia vera and related disorders.7
In summary, the study by Boggon and colleagues is an important advance that should facilitate the development of Jak inhibitors. Exploiting the unique aspects of Jak3 structure can hopefully help in the generation of more selective inhibitors. Among the Jaks, Jak3 is most closely related to Jak2, which is important for erythropoietin and thrombopoietin signaling. Minimizing the effect of a Jak3 inhibitor on Jak2 could be of clinical benefit. Conversely, this information might be helpful in generating selective inhibitors of other Jaks. For instance, Tyk2, which is critical for IL-12 signaling, may be a useful target in diseases characterized by T-helper 1 (Th1)–mediated pathology, whereas targeting mutant Jak2 molecules could be of use in the treatment of myeloproliferative disorders. While the solution of the structures of the individual Jak domains is important, defining the structure of an intact Jak molecule will ultimately be needed to understand how these critical kinases are regulated. Understanding precisely how the pseudokinase domain regulates catalytic activity might offer unique opportunities for intervention. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal