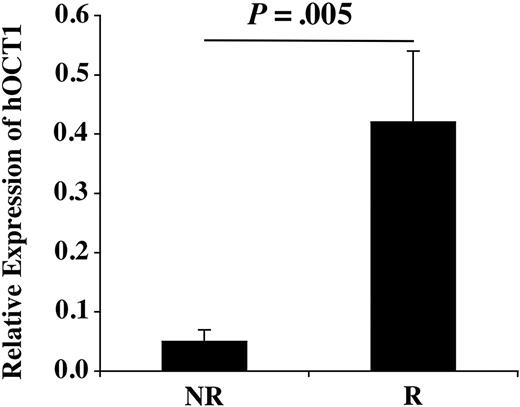
The pre-imatinib hOCT1 expression level in nonresponders (NRs) and responders (Rs).
The pre-imatinib hOCT1 expression level in nonresponders (NRs) and responders (Rs).
Imatinib is a substrate for the adenosine triphosphate–binding cassette (ABC) transporters ABCB11-3 and ABCG24,5 ; however, it is unclear whether these transporters influence patients' responses to imatinib. Thomas et al6 recently reported on the active transport of imatinib into cells by the human organic cation transporter 1 (hOCT1). They proposed that in patients with chronic myeloid leukemia (CML), the differential expression of hOCT1 and other drug transporters might be a critical determinant of intracellular drug levels, and hence influence response to imatinib.
To investigate hOCT1, ABCA2, ABCG2, and ABCB1 as potential sources of primary cytogenetic resistance to imatinib, we examined their expression in a cohort of 30 patients with CML. Patients were defined as responders (Rs) if they had achieved a complete cytogenetic response to imatinib within the first year of therapy (n = 15), and nonresponders (NRs) if they had remained at least 65% Philadelphia-chromosome positive by cytogenetics during the first 10 months of imatinib treatment (n = 15). Rs and NRs were closely matched for sex, disease phase at time of starting imatinib, and age at diagnosis (P = .42); although median time from diagnosis to starting imatinib was lower for Rs (20 months; range 5.3-55.5 months) than NRs (41.7 months; range 6.8-101.8 months). All patients had had bone marrow (BM) mononuclear cells (MNCs) cryopreserved immediately prior to imatinib therapy, and all NRs had BM MNCs stored after 9 to 15 months of imatinib treatment. Four normal BM MNC samples were obtained from AllCells (Berkeley, CA).
BM MNC gene expression was assayed by quantitative real-time polymerase chain reaction (PCR) and normalized for Abl-b expression. We found that baseline expression of hOCT1 in CML patients was variable and not significantly different from healthy bone marrow donors, though the number of normal samples was small. Interestingly, the mean pre-imatinib expression level in NRs was one eighth that seen in Rs (P = .005) (Figure 1). On imatinib, 6 NRs did show a further 2-fold decrease in expression compared with baseline, though this was not consistent across the group (Table 1).
Summary of the characteristics of individual NRs, their gene expression changes with time on imatinib, and disease progression
Patient ID . | Disease phase . | hOCT1 . | ABCA2 . | ABCG2 . | ABCB1 . | KM . | Days to progression . | Disease progression . |
|---|---|---|---|---|---|---|---|---|
| 1 | CP | – | – | ∼ | ∼ | nil | 1493 | CE |
| 2 | CP | – | – | + | + | nil | NA | none |
| 3 | AP | – | – | ∼ | + | nil | 987 | CE |
| 4 | CP | – | – | + | ∼ | nil | NA | none |
| 5 | CP | – | – | ∼ | ∼ | nil | NA | none |
| 6 | CP | + | ∼ | + | ∼ | G250E | 167 | AP |
| 7 | CP | – | – | – | ∼ | nil | 297 | MBC |
| 8 | CP | + | – | – | – | nil | NA | none |
| 9 | CP | ∼ | + | ∼ | – | nil | NA | none |
| 10 | CP + CE | + | + | + | + | nil | NA | none |
| 11 | CP | + | + | ∼ | ∼ | nil | NA | none |
| 12 | CP | + | + | ∼ | ∼ | nil | NA | none |
| 13 | CP | ∼ | + | + | ∼ | nil | 941 | CE |
| 14 | CP | ∼ | ∼ | ∼ | ∼ | nil | NA | none |
| 15 | CP + CE | + | + | ∼ | ∼ | Y253F | 1076 | CE |
Patient ID . | Disease phase . | hOCT1 . | ABCA2 . | ABCG2 . | ABCB1 . | KM . | Days to progression . | Disease progression . |
|---|---|---|---|---|---|---|---|---|
| 1 | CP | – | – | ∼ | ∼ | nil | 1493 | CE |
| 2 | CP | – | – | + | + | nil | NA | none |
| 3 | AP | – | – | ∼ | + | nil | 987 | CE |
| 4 | CP | – | – | + | ∼ | nil | NA | none |
| 5 | CP | – | – | ∼ | ∼ | nil | NA | none |
| 6 | CP | + | ∼ | + | ∼ | G250E | 167 | AP |
| 7 | CP | – | – | – | ∼ | nil | 297 | MBC |
| 8 | CP | + | – | – | – | nil | NA | none |
| 9 | CP | ∼ | + | ∼ | – | nil | NA | none |
| 10 | CP + CE | + | + | + | + | nil | NA | none |
| 11 | CP | + | + | ∼ | ∼ | nil | NA | none |
| 12 | CP | + | + | ∼ | ∼ | nil | NA | none |
| 13 | CP | ∼ | + | + | ∼ | nil | 941 | CE |
| 14 | CP | ∼ | ∼ | ∼ | ∼ | nil | NA | none |
| 15 | CP + CE | + | + | ∼ | ∼ | Y253F | 1076 | CE |
KM indicates kinase mutation after imatinib; CP, chronic phase; –, at least a halving in gene expression with time on imatinib; CE, clonal evolution; +, at least a doubling in gene expression with time on imatinib; AP, accelerated phase; MBC, myeloid blast crisis; and CP + CE, chronic phase with evidence of clonal evolution. ∼ indicates no change in gene expression with time on imatinib; nil, no kinase mutation detected; NA, not applicable; and none, no disease progression during follow up.
In contrast to hOCT1, the pre-imatinib expression levels of ABCA2, ABCG2, and ABCB1 were similar for Rs, NRs, and normal BM. After imatinib exposure, 6, 5, and 3 NRs showed at least a doubling of ABCA2, ABCG2, and ABCB1 expression, respectively (Table 1), but when the whole group of NRs was considered, these results were not statistically significant.
All NRs were screened for kinase mutations (KMs) after imatinib; 2 patients had a single KM each (Table 1).
Since hOCT1 actively transports imatinib into cells, patients with low baseline expression of hOCT1 may be unable to achieve adequate intracellular concentrations of imatinib, and hence fail to achieve a cytogenetic response. Although our study is small, our observations add weight to Thomas et al's6 proposal that differential expression of hOCT1 may affect patients' responses to imatinib. We believe that further work is warranted to explore the interaction of hOCT1 and other drug transporters as a cause of primary cytogenetic resistance to imatinib.
Imatinib and hOCT1: implications for drug resistance and interactions
In our study,1 we showed that imatinib was a substrate for human organic cation transporter 1 (hOCT1) and suggested that the balance between the expression of efflux (adenosine triphosphate–binding cassette transporter B1 [ABCB1]) and influx (hOCT1) transporters might determine the intracellular levels and hence the response to imatinib. Despite the small numbers studied, the study by Crossman et al is certainly in accordance with this. Indeed, we have also recently found in a larger patient sample (n = 67) that expression of hOCT1 varies between responders and nonresponders (Wang et al, manuscript in preparation).
There are many mechanisms for resistance to chemotherapy: with respect to transporters, the focus has been on overexpression of efflux transporters, in particular ABCB1.2 This has led to studies where ABCB1 inhibitors such as verapamil have been used in combination with chemotherapeutic agents to increase intracellular drug levels, but unfortunately without much success.2 The finding that down-regulation of influx transporters such as hOCT1 may be another mechanism for resistance is novel, and may also explain why previous studies that have attempted to modulate transporter function have not been successful, since many of these drugs lack specificity of inhibition. For example, verapamil inhibits not only ABCB1 but also hOCT1.
Before we can use this information therapeutically to improve therapy with imatinib and possibly with the newer analogs currently in development, many questions need to be answered. We do not know what determines variable expression of hOCT1; possibilities include that expression is genetically determined, related to the disease process itself, or due to concurrent drug therapy (including with imatinib). The worst scenario would theoretically be a patient who has low expression of hOCT1 but high expression of ABCB1 and ABCG2.
The evidence that imatinib is a substrate for several transporters also provides a mechanistic basis with which to predict interactions with imatinib, which may affect its efficacy. For example, the known interaction of imatinib with St Johns Wort3 is likely to be due to both induction of cytochrome P450 3A4 (CYP3A4, which metabolizes imatinib) in the liver but also of ABCB1 in CML cells, both of which are likely to reduce intracellular imatinib levels. Furthermore, the recent finding that HIV protease inhibitors can inhibit hOCT14 suggests that this may be another mechanism for interactions with imatinib. We therefore strongly agree with Crossman et al that this is an important area for further research, and may offer novel insights into how to improve the effectiveness of drugs such as imatinib.
Correspondence: M. Pirmohamed, Department of Pharmacology, The University of Liverpool, Ashton Street, Liverpool, L69 3GE, United Kingdom; e-mail: munirp@liv.ac.uk.
L.C.C. is a recipient of a Clinical Research Fellowship from the Leukaemia Research Fund of Great Britain. M.W.N.D. is a Junior Faculty Scholar of the American Society of Hematology. This work was supported by the Howard Hughes Medical Institute (B.J.D.), and grants from The Leukemia and Lymphoma Society (B.J.D.), the T. J. Martell Foundation (B.J.D.), and the Burroughs Wellcome Fund (B.J.D.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal