Abstract
The average results of some laboratory measurements, including the hemoglobin, mean corpuscular volume (MCV), serum transferrin saturation (TS), serum ferritin, and white blood cell count of African-Americans differ from those of whites. Anonymized samples and laboratory data from 1491 African-American and 31 005 white subjects, approximately equally divided between men and women, were analyzed. The hematocrit, hemoglobin, MCV, TS, and white blood cell counts of African-Americans were lower than those of whites; serum ferritin levels were higher. When iron-deficient patients were eliminated from consideration the differences in hematocrit, hemoglobin, and MCV among women were slightly less. The -3.7-kilobase α-thalassemia deletion accounted for about one third of the difference in the hemoglobin levels of African-Americans and whites and neither sickle trait nor elevated creatinine levels had an effect. Among all subjects, 19.8% of African-American women would have been classified as “anemic” compared with 5.3% of whites. Among men, the figures were 17.7% and 7.6%. Without iron-deficient or thalassemic subjects, the difference had narrowed to 6.1% and 2.77% and to 4.29% and 3.6%, respectively. Physicians need to take into account that the same reference standards for hemoglobin, hematocrit, MCV, and TS and the white blood cell count do not apply to all ethnic groups. (Blood. 2005;106:740-745)
Introduction
While it is customary to apply the same reference ranges to patients with diverse ancestral origins, it has been known for some time that there are differences, particularly between “normal” values obtained from subjects with European or African ancestry. For example, compared to whites, African-Americans appear to have lower serum transferrin saturation (TS), higher serum ferritin levels,1-3 lower bilirubin levels,4 and lower leukocyte counts.5 Perhaps most importantly, the average hemoglobin level, hematocrit, and mean corpuscular volume (MCV) are lower in African-Americans than in whites.
Earlier studies documenting the difference in normal hemoglobin levels in African-American men, women, and children compared with their white counterparts were reviewed in detail in 1992,2 and additional studies have appeared since.6,7 Indeed, it has been suggested that different reference ranges need to be considered for these 2 groups,5 but the validity of some of the studies has been challenged.8
We have now had the opportunity to extend these studies on a patient cohort even larger than that provided by the National Health and Nutrition Examination Survey (NHANES)-based studies,2,9 and have focused on possible explanations for the low hemoglobin level and MCV of African-Americans as compared with whites.
Patients, materials, and methods
Human subjects
Blood was obtained with informed consent in a study approved by the Kaiser Permanente and Scripps Research Institute institutional review boards. The composition and demographics of the patient population, members of a health plan who availed themselves of the opportunity to undergo a screening health examination, has been described previously.7,10,11 The ethnic origin of each subject was based on self-identification. No samples were used from subjects who declared mixed or Hispanic ancestry. No data regarding family income or occupation were available, but the questionnaire completed by all of the subjects provided information regarding the level of formal education that each subject had achieved. Determinations of the hemoglobin level, hematocrit, MCV, white blood cell count, a flow-cytometric differential, serum ferritin, and TS were a routine part of the appraisal of these patients. Consequently, about 99% of the hemoglobin, hematocrit, MCV, white blood cell count, differential, and TS values were recorded, and serum ferritin values were available in about 95% of the subjects.
Samples from 292 African-American women and 306 African-American men were genotyped for the α-globin -3.7-kilobase (kb) deletion and the mutations in the β-globin chain for hemoglobin S. Age- and sex-matched samples from subjects declaring “white” ancestry served as controls for this subset of patients. Since abundant numbers of such samples were available, a large excess of white control subjects was used for comparison of red-cell parameters with African-American subjects. DNA samples from 155 white subjects were genotyped for the α-globin -3.7-kb deletion.
Mutation detection
Polymerase chain reaction (PCR) amplification of the region containing the α-thalassemic -3.7-kb deletion was performed using the oligonucleotide primers P54 and P55 according to the method of Oron-Karni et al12 with minor modifications. The β-globin sickle mutation was detected in a PCR-amplified 110-base pair (bp) fragment of the β-globin gene containing the sickle mutation using primers described by Wittwer et al.13
Statistical analysis
Differences between white and African-American subjects were evaluated using Student t test, assuming that all parameters were normally distributed except for the ferritin, the distribution of which was assumed to be log normal.
Results
Hematologic measurements on the entire patient group
The results of hematologic measurements on the entire group studied are shown in Tables 1 and 2. It is apparent that the average hemoglobin and hematocrit levels, white blood cell count and absolute granulocyte count, and TS are lower in the African-American than in the white group, and that the serum ferritin and absolute lymphocyte count is higher in the African-American group. The differences were all highly statistically significant except for the serum ferritin values in the African-American women. As shown in Table 3 it is notable that there was no relationship between hemoglobin or MCV measurements and educational status, implying that these differences were unrelated to socioeconomic status.
The blood hemoglobin concentration, hematocrit level, MCV, serum ferritin concentration, and TS of all African-American and white subjects
. | No. . | Hematocrit, % . | Hemoglobin, g/dL . | MCV, fL . | TS, % . | Ferriting μg/L, mean (95% CI)* . |
|---|---|---|---|---|---|---|
| African-American women | 760 | 37.54 ± 0.12 | 12.70 ± 0.04 | 86.79 ± 0.24 | 20.54 ± 0.33 | 59.15 (54.89, 63.75) |
| White women | 15 624 | 39.28 ± 0.02 | 13.49 ± 0.01 | 90.50 ± 0.04 | 24.11 ± 0.08 | 54.69 (53.94, 55.46) |
| African-American men | 733 | 42.55 ± 0.12 | 14.45 ± 0.04 | 87.65 ± 0.21 | 26.03 ± 0.34 | 142.20 (133.86, 151.06) |
| White men | 15 405 | 43.23 ± 0.02 | 14.93 ± 0.01 | 90.68 ± 0.04 | 28.22 ± 0.08 | 115.55 (114.05, 117.06) |
. | No. . | Hematocrit, % . | Hemoglobin, g/dL . | MCV, fL . | TS, % . | Ferriting μg/L, mean (95% CI)* . |
|---|---|---|---|---|---|---|
| African-American women | 760 | 37.54 ± 0.12 | 12.70 ± 0.04 | 86.79 ± 0.24 | 20.54 ± 0.33 | 59.15 (54.89, 63.75) |
| White women | 15 624 | 39.28 ± 0.02 | 13.49 ± 0.01 | 90.50 ± 0.04 | 24.11 ± 0.08 | 54.69 (53.94, 55.46) |
| African-American men | 733 | 42.55 ± 0.12 | 14.45 ± 0.04 | 87.65 ± 0.21 | 26.03 ± 0.34 | 142.20 (133.86, 151.06) |
| White men | 15 405 | 43.23 ± 0.02 | 14.93 ± 0.01 | 90.68 ± 0.04 | 28.22 ± 0.08 | 115.55 (114.05, 117.06) |
Values are given as the mean ± 1 standard error (SE).
Geometric mean (lower, upper 95% confidence limit).
The white blood cell count, granulocyte count, and lymphocyte count of all African-American and white subjects
. | WBCs, × 109/L . | Granulocytes, × 109/L . | Lymphocytes, × 109/L . |
|---|---|---|---|
| African-American women | 6.14 ± 0.07 | 3.37 ± 0.06 | 2.14 ± 0.02 |
| White women | 6.76 ± 0.02 | 4.06 ± 0.01 | 1.96 ± 0.05 |
| African-American men | 5.74 ± 0.06 | 3.12 ± 0.05 | 1.96 ± 0.02 |
| White men | 6.54 ± 0.01 | 3.92 ± 0.01 | 1.82 ± 0.06 |
. | WBCs, × 109/L . | Granulocytes, × 109/L . | Lymphocytes, × 109/L . |
|---|---|---|---|
| African-American women | 6.14 ± 0.07 | 3.37 ± 0.06 | 2.14 ± 0.02 |
| White women | 6.76 ± 0.02 | 4.06 ± 0.01 | 1.96 ± 0.05 |
| African-American men | 5.74 ± 0.06 | 3.12 ± 0.05 | 1.96 ± 0.02 |
| White men | 6.54 ± 0.01 | 3.92 ± 0.01 | 1.82 ± 0.06 |
Data are expressed as mean ± SE.
WBCs indicates white blood cells.
The hemoglobin level and MCV of African-American and white subjects, stratified by education level achieved
Sex, race, and educational status . | Percent . | Hemoglobin, g/dL, mean ± SE . | MCV, fL, mean ± SE . |
|---|---|---|---|
| Women | |||
| African-American | |||
| Postgraduate degree | 10.88 | 12.77 ± 0.13 | 86.70 ± 0.77 |
| College degree | 25.56 | 12.70 ± 0.08 | 87.90 ± 0.39 |
| Some college | 44.30 | 12.65 ± 0.07 | 86.39 ± 0.39 |
| High school diploma or GED | 13.63 | 12.72 ± 0.12 | 85.93 ± 0.70 |
| Some high school | 3.67 | 12.88 ± 0.20 | 87.43 ± 1.12 |
| Elementary school | 0.79 | 13.48 ± 0.34 | 88.63 ± 2.06 |
| Unknown | 1.18 | 12.57 ± 0.27 | 86.44 ± 1.63 |
| White | |||
| Postgraduate degree | 15.36 | 13.43 ± 0.02 | 90.63 ± 0.09 |
| College degree | 23.00 | 13.44 ± 0.02 | 90.43 ± 0.08 |
| Some college | 37.16 | 13.52 ± 0.01 | 90.47 ± 0.06 |
| High school diploma or GED | 19.29 | 13.52 ± 0.02 | 90.52 ± 0.09 |
| Some high school | 3.44 | 13.53 ± 0.04 | 90.64 ± 0.21 |
| Elementary school | 0.60 | 13.54 ± 0.11 | 90.04 ± 0.52 |
| Unknown | 1.15 | 13.58 ± 0.08 | 91.12 ± 0.37 |
| Men | |||
| African-American | |||
| Postgraduate degree | 11.35 | 14.49 ± 0.12 | 87.28 ± 0.62 |
| College degree | 25.31 | 14.49 ± 0.09 | 88.06 ± 0.38 |
| Some college | 43.37 | 14.45 ± 0.07 | 87.34 ± 0.34 |
| High school diploma or GED | 15.18 | 14.34 ± 0.10 | 88.13 ± 0.55 |
| Some high school | 3.15 | 14.47 ± 0.28 | 87.69 ± 1.17 |
| Elementary school | 0.55 | 14.48 ± 0.69 | 87.20 ± 2.90 |
| Unknown | 1.09 | 14.41 ± 0.29 | 88.25 ± 1.74 |
| White | |||
| Postgraduate degree | 21.86 | 14.89 ± 0.02 | 90.73 ± 0.07 |
| College degree | 28.43 | 14.97 ± 0.02 | 90.55 ± 0.07 |
| Some college | 31.01 | 14.96 ± 0.02 | 90.63 ± 0.07 |
| High school diploma or GED | 13.67 | 14.88 ± 0.03 | 90.88 ± 0.11 |
| Some high school | 3.10 | 14.80 ± 0.05 | 90.84 ± 0.22 |
| Elementary school | 0.71 | 14.49 ± 0.12 | 90.59 ± 0.48 |
| Unknown | 1.23 | 14.92 ± 0.09 | 91.52 ± 0.34 |
Sex, race, and educational status . | Percent . | Hemoglobin, g/dL, mean ± SE . | MCV, fL, mean ± SE . |
|---|---|---|---|
| Women | |||
| African-American | |||
| Postgraduate degree | 10.88 | 12.77 ± 0.13 | 86.70 ± 0.77 |
| College degree | 25.56 | 12.70 ± 0.08 | 87.90 ± 0.39 |
| Some college | 44.30 | 12.65 ± 0.07 | 86.39 ± 0.39 |
| High school diploma or GED | 13.63 | 12.72 ± 0.12 | 85.93 ± 0.70 |
| Some high school | 3.67 | 12.88 ± 0.20 | 87.43 ± 1.12 |
| Elementary school | 0.79 | 13.48 ± 0.34 | 88.63 ± 2.06 |
| Unknown | 1.18 | 12.57 ± 0.27 | 86.44 ± 1.63 |
| White | |||
| Postgraduate degree | 15.36 | 13.43 ± 0.02 | 90.63 ± 0.09 |
| College degree | 23.00 | 13.44 ± 0.02 | 90.43 ± 0.08 |
| Some college | 37.16 | 13.52 ± 0.01 | 90.47 ± 0.06 |
| High school diploma or GED | 19.29 | 13.52 ± 0.02 | 90.52 ± 0.09 |
| Some high school | 3.44 | 13.53 ± 0.04 | 90.64 ± 0.21 |
| Elementary school | 0.60 | 13.54 ± 0.11 | 90.04 ± 0.52 |
| Unknown | 1.15 | 13.58 ± 0.08 | 91.12 ± 0.37 |
| Men | |||
| African-American | |||
| Postgraduate degree | 11.35 | 14.49 ± 0.12 | 87.28 ± 0.62 |
| College degree | 25.31 | 14.49 ± 0.09 | 88.06 ± 0.38 |
| Some college | 43.37 | 14.45 ± 0.07 | 87.34 ± 0.34 |
| High school diploma or GED | 15.18 | 14.34 ± 0.10 | 88.13 ± 0.55 |
| Some high school | 3.15 | 14.47 ± 0.28 | 87.69 ± 1.17 |
| Elementary school | 0.55 | 14.48 ± 0.69 | 87.20 ± 2.90 |
| Unknown | 1.09 | 14.41 ± 0.29 | 88.25 ± 1.74 |
| White | |||
| Postgraduate degree | 21.86 | 14.89 ± 0.02 | 90.73 ± 0.07 |
| College degree | 28.43 | 14.97 ± 0.02 | 90.55 ± 0.07 |
| Some college | 31.01 | 14.96 ± 0.02 | 90.63 ± 0.07 |
| High school diploma or GED | 13.67 | 14.88 ± 0.03 | 90.88 ± 0.11 |
| Some high school | 3.10 | 14.80 ± 0.05 | 90.84 ± 0.22 |
| Elementary school | 0.71 | 14.49 ± 0.12 | 90.59 ± 0.48 |
| Unknown | 1.23 | 14.92 ± 0.09 | 91.52 ± 0.34 |
GED indicates General Equivalency Diploma.
Red-cell parameters of African-American and age-matched white patients
In the overall patient population, the average age of the white men was 57.6 years, African-American men was 51.0 years, white women was 57.6 years, and African-American women was 49.8 years. This difference might have some effect in accounting for the differences observed in the overall population, particularly on hemoglobin levels of women. Accordingly, we age-matched a subset of patients for further analysis. Figure 1 compares the hemoglobin concentration, MCV, TS, and serum ferritin of all of the age-matched African-American and white subjects. Among age-matched subjects of the same sex, the average hemoglobin level was higher in whites (0.72 g/dL in women; 0.58 g/dL in men) as was the hematocrit level (1.55% in women; 0.92% in men). The average MCV was also lower in African-Americans (2.99 fl in women; 2.72 fl in men).
Hematologic parameters of age-matched, genotyped African-American and age-matched white clinic patients, excluding subjects with iron deficiency
To determine the effect of iron-deficient hematopoiesis, due either to iron deficiency or inflammation on parameters of red cell homeostasis, the data were reanalyzed in age-matched subjects, excluding those with either a TS of less than 16% or a serum ferritin level of less than 10 μg per. The few cases in which both measurements were not available were also excluded. When patients with possible iron deficiency or iron-deficient erythropoiesis were excluded in this way the difference between African-American and white women narrowed moderately, whereas there was little change among the men (Figure 1). The average hemoglobin of African-American and white women of the iron-sufficient group had narrowed to 0.60 g/dL whereas that of the men remained essentially unchanged at 0.56 g/dL. The difference in the average MCV of African-American and white women narrowed slightly to 2.69 fl, whereas that of men narrowed to 2.55 fl.
The blood hemoglobin concentration, MCV, TS, and serum ferritin of 598 African-American men and women and more than 12 000 age-matched white controls attending a Health Appraisal Clinic. The African-American subjects profiled in the figure were attending a Health Appraisal Clinic and were genotyped for α-thalassemia and hemoglobin S. The error bars represent one standard error of the mean. (A) All subjects without exclusions. (B) Subjects after removing those with iron deficiency (see “Patients, materials, and methods”). AA indicates African-American. Error bars indicate standard error of the mean (SEM).
The blood hemoglobin concentration, MCV, TS, and serum ferritin of 598 African-American men and women and more than 12 000 age-matched white controls attending a Health Appraisal Clinic. The African-American subjects profiled in the figure were attending a Health Appraisal Clinic and were genotyped for α-thalassemia and hemoglobin S. The error bars represent one standard error of the mean. (A) All subjects without exclusions. (B) Subjects after removing those with iron deficiency (see “Patients, materials, and methods”). AA indicates African-American. Error bars indicate standard error of the mean (SEM).
The effect of α-thalassemia on hematologic parameters
Gene frequency of the 3.7-kb deletion form of α-thalassemia. Of the 598 African-American subjects who were genotyped, 414 (69.2%) were wild type (ie, 4 copies of the alpha locus were present), 166 (27.8%) were heterozygous, and 18 (3.0%) were homozygous for the 3.7-kb deletion. The allele frequency for the deletion, then, was 0.169, and the predicted number of homozygotes according to the Hardy-Weinberg equilibrium was 17.1, very close to the actual number of 18 found. Among 155 white subjects only 1 of the 310 alleles examined was found to bear the mutation.
Phenotypic effect of the 3.7-kb deletion form of α-thalassemia.Table 4 summarizes the relationship between the genotype and the average hemoglobin concentration of the blood, hematocrit, and MCV of the iron-sufficient African-American subjects and the age-matched white controls. It is apparent that the presence of the α locus deletion influences the hemoglobin and MCV. In performing the statistical analysis, values for the homozygotes and heterozygotes for the deletion were pooled and compared with the wild-type (αα/αα) genotype because of the small number of homozygotes for the α locus deletion.
Effect of the α-thalassemia genotypes on red cell measurements of non-iron-deficient African-American subjects and non-iron-deficient white controls
Sex and genotype . | No. . | Hemoglobin, g/dL, mean ± SE . | MCV, fL, mean ± SE . |
|---|---|---|---|
| F | |||
| −α3.7/−α3.7 | 6 | 12.02 ± 0.224 | 72.33 ± 1.260 |
| αα/−α3.7 | 42 | 12.58 ± 0.145 | 84.20 ± 0.544 |
| αα/αα | 129 | 13.12 ± 0.081 | 90.26 ± 0.391 |
| White controls | 5780 | 13.44 ± 0.013 | 89.94 ± 0.0618 |
| M | |||
| −α3.7/−α3.7 | 7 | 13.60 ± 0.315 | 75.71 ± 2.275 |
| αα/−α3.7 | 79 | 14.25 ± 0.122 | 85.05 ± 0.526 |
| αα/αα | 172 | 14.72 ± 0.077 | 89.59 ± 0.357 |
| White controls | 6240 | 15.06 ± 0.012 | 90.11 ± 0.0547 |
Sex and genotype . | No. . | Hemoglobin, g/dL, mean ± SE . | MCV, fL, mean ± SE . |
|---|---|---|---|
| F | |||
| −α3.7/−α3.7 | 6 | 12.02 ± 0.224 | 72.33 ± 1.260 |
| αα/−α3.7 | 42 | 12.58 ± 0.145 | 84.20 ± 0.544 |
| αα/αα | 129 | 13.12 ± 0.081 | 90.26 ± 0.391 |
| White controls | 5780 | 13.44 ± 0.013 | 89.94 ± 0.0618 |
| M | |||
| −α3.7/−α3.7 | 7 | 13.60 ± 0.315 | 75.71 ± 2.275 |
| αα/−α3.7 | 79 | 14.25 ± 0.122 | 85.05 ± 0.526 |
| αα/αα | 172 | 14.72 ± 0.077 | 89.59 ± 0.357 |
| White controls | 6240 | 15.06 ± 0.012 | 90.11 ± 0.0547 |
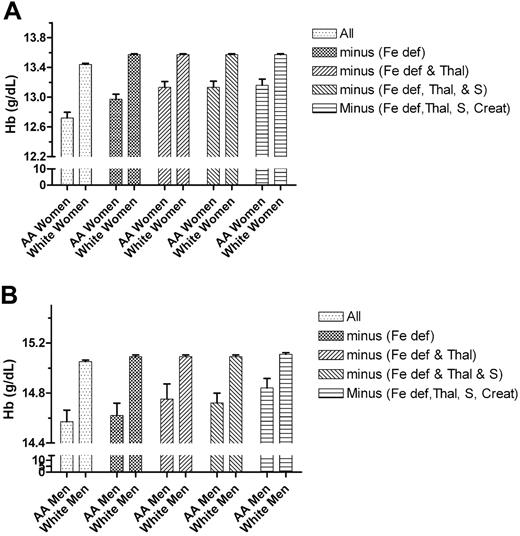
We then compared the hemoglobin level, hematocrit, and MCV of those African-American patients who had no evidence of iron-deficient erythropoiesis or of α-thalassemia mutations with age-matched white controls. Even with these exclusions, a highly significant difference in these parameters could be demonstrated. The further removal of patients with sickle trait produced no change in the values. The effect of successively removing from the cohort, first patients with iron deficiency, then those with thalassemia, and finally those with sickle trait is shown in Figure 2.
The effect of sequentially removing subjects with iron deficiency, the α-thalassemia -3.7-kb allele, sickle trait, and serum creatinine levels of more than 1.4 mg/dL on the hemoglobin level (Hb) of African-American men and women and of age- and sex-matched white controls. Top graph, women; bottom graph, men. Fe def indicates iron deficiency; Thal, α-thalassemia -3.7-kb allele; S, sickle trait; and Creat, serum creatinine levels of more than 1.4 mg/dL. Error bars indicate SEM.
The effect of sequentially removing subjects with iron deficiency, the α-thalassemia -3.7-kb allele, sickle trait, and serum creatinine levels of more than 1.4 mg/dL on the hemoglobin level (Hb) of African-American men and women and of age- and sex-matched white controls. Top graph, women; bottom graph, men. Fe def indicates iron deficiency; Thal, α-thalassemia -3.7-kb allele; S, sickle trait; and Creat, serum creatinine levels of more than 1.4 mg/dL. Error bars indicate SEM.
Effect of iron deficiency, α-thalassemia, and sickle trait on the prevalence of “anemia”
As shown in Table 5, nearly 20% of the African-American women in the entire population studied would have been judged anemic based on a hemoglobin cutoff of 12 g/dL. This is almost 4 times the percentage of white women judged to be anemic based on this criterion. In the case of men, the percentage with hemoglobin levels of below 13.5 g/dL was more than twice as great in the African-American as in the white population. Removing iron-insufficient subjects reduced the number of “anemic” African-American women by almost a third, but had a similar effect on white women. The elimination of African-American women carrying the α-thalassemia deletion reduced the number of “anemic” women to about one half, but the number was still in excess of 6%, more than twice the number of white women in this category. A similar effect was documented among men. Removing men with plasma creatine levels of more than 1.4 mg/dL had some effect, but the differences between whites and African-Americans were retained.
Percentage of subjects classified as “anemic” based on blood hemoglobin concentrations of less than 12 g/dL for women and less than 13.5 g/dL for men
Group . | African-American women, % (no.) . | White women, % (no.) . | African-American men, % (no.) . | White men, % (no.) . |
|---|---|---|---|---|
| A: All subjects | 19.79 (758) | 5.25 (15 612) | 17.74 (733) | 7.61 (15 393) |
| B: Genotyped African-Americans and matched controls | 19.72 (284) | 5.50 (5 780) | 13.80 (310) | 4.39 (6 240) |
| C: Group B, minus those with low ferritin and low TS | 13.90 (187) | 2.84 (4 258) | 11.36 (264) | 3.83 (5 373) |
| D: Group C, minus those with α-thalassemia | 6.5 (128) | NA | 8.14 (172) | NA |
| E: Group D, minus those with sickle trait | 6.60 (122) | NA | 7.32 (164) | NA |
| F: Group E, minus those with a creatinine level greater than 1.4 mg/dL | 6.09 (111) | NA | 4.29 (140) | NA |
| G: Group C, minus those with a creatinine level greater than 1.4 mg/dL | NA | 2.77 (4 110) | NA | 3.56 (5 058) |
Group . | African-American women, % (no.) . | White women, % (no.) . | African-American men, % (no.) . | White men, % (no.) . |
|---|---|---|---|---|
| A: All subjects | 19.79 (758) | 5.25 (15 612) | 17.74 (733) | 7.61 (15 393) |
| B: Genotyped African-Americans and matched controls | 19.72 (284) | 5.50 (5 780) | 13.80 (310) | 4.39 (6 240) |
| C: Group B, minus those with low ferritin and low TS | 13.90 (187) | 2.84 (4 258) | 11.36 (264) | 3.83 (5 373) |
| D: Group C, minus those with α-thalassemia | 6.5 (128) | NA | 8.14 (172) | NA |
| E: Group D, minus those with sickle trait | 6.60 (122) | NA | 7.32 (164) | NA |
| F: Group E, minus those with a creatinine level greater than 1.4 mg/dL | 6.09 (111) | NA | 4.29 (140) | NA |
| G: Group C, minus those with a creatinine level greater than 1.4 mg/dL | NA | 2.77 (4 110) | NA | 3.56 (5 058) |
NA indicates not applicable.
Discussion
As modern humans emerged from Africa and encountered new environmental pressures in Europe and Asia, numerous adaptive changes occurred in the gene pool. Some single gene mutations that occur largely among persons with African ancestry, such as the sickle hemoglobinopathy and G6PD deficiency are well known; presumably, these mutations were selected by the pressure exerted by malarial infection. But there are also quantitative differences in the results of various blood tests that are less widely appreciated by physicians and that may influence, at least in some cases, the interpretation of the standard laboratory test used in hematology and other fields of medicine.
In a large population of African-American and white subjects undergoing health screening, we found that whites had higher hematocrit levels, higher hemoglobin levels, higher MCVs, higher TSs, and lower serum ferritin levels than did African-Americans. The white blood cell counts of African-Americans were lower, and this difference was due principally to lower granulocyte counts, whereas absolute lymphocyte counts were actually higher in African-Americans. These data agreed with the observations of others (see Perry et al2 for a detailed review of the hemoglobin data). Although it has been proposed that the difference in hemoglobin levels might be due primarily to socioeconomic and nutritional factors,8 most studies have suggested that there are other causes, very possibly genetic, that account for the difference.2,6,14-16 Quite specifically, Perry et al2 have provided evidence that the hemoglobin difference is not due to iron deficiency. Elevated serum ferritin levels have been found in African-Americans in previous studies.1-3 While the cause for this difference between African-American and white subjects is not fully understood, it is likely to have a genetic basis, since familial segregation of the ferritin levels has been demonstrated among Africans.17
We applied very stringent criteria to eliminate iron-deficient patients from consideration, viz, either a ferritin level of less than 10 ng/mL or a TS of less than 16%. By applying such criteria we undoubtedly also excluded some patients who were not iron deficient, very possibly some with iron-deficient erythropoiesis as occurs in the anemia of chronic disease. We chose this course because even the latter patients should not be included in attempting to determine what the normal average hemoglobin concentration is in the population. Not surprisingly, removal of iron-deficient patients from consideration had a much greater effect in women than in men, and a somewhat greater effect in African-American than in white women. The higher prevalence of iron-deficiency anemia in African-American women is not surprising in view of the fact that leiomyoma are more common in this group,18 as is the prevalence of heavy menstrual bleeding.19 But clearly much of the difference between African-Americans and whites was still present when these patients had been eliminated from consideration (Figure 2).
The thalassemic -3.7 kb α-chain deletion is very common in the African-American population.20-22 However, there are very few data regarding its effect on sex-specific hematologic findings23 and the data that are available are largely based on globin-chain synthesis, a method that does not allow unequivocal differentiation of the genotypes.22,24 Because of the paucity of data it has previously not been clear whether or how much this common polymorphism contributes to the difference in hemoglobin levels between people of European and African ancestry. Another polymorphism that may play a role is that of hemoglobin S, since in the heterozygous state this mutation has been reported to be associated with slightly lower hemoglobin levels.25,26
Methods are now available for accurately genotyping large numbers of patients for these polymorphisms using PCR-based technology, and we have had the opportunity of investigating the factors that might be responsible for the difference in hemoglobin levels of African-Americans and whites. Using this technology, we documented a gene frequency of 0.169 for the α-globin deletion, remarkably close to the value of 0.156 found by Dozy et al20 25 years ago using Southern blotting on 211 African-Americans. Serjeant et al27 found a gene frequency of 0.178 among 246 Jamaican children with West African ancestry who did not have hemoglobins C, S, or β-thalassemia. Lower estimates of the gene frequency of approximately 0.0722 and of 0.1121 were made by measuring globin-chain synthesis. Presumably, the mutation is not fully penetrant in this regard.
Previously, with the limited number of patients studied and the imprecision of the globin-chain synthesis technology we were not able to show a statistically significant difference in hemoglobin levels, although there was an effect of the α-thalassemia mutation on the MCV.22 We now can present a more comprehensive picture of the effect of the α-thalassemia mutation on the blood counts of heterozygotes and homozygotes. Quite clearly, as shown in Table 4, it produces an effect both on hemoglobin values and on the MCV. In the present study we were able to demonstrate that removing from consideration patients homozygous or heterozygous for the 3.7 kb α-globin locus deletion did narrow the difference between African-Americans and whites (Figure 2).
Two studies have suggested that patients with sickle trait have lower hemoglobin levels than controls.25,26 We did not see such an effect when the contribution of the α-thalassemia mutation is taken into account. It is notable in this regard that in our previous study22 we found that the sickle mutation was more common in patients with α-thalassemia genes. We suggested that this might be the case because persons with a high percentage of African ancestry would be more likely to carry both genes than those with a high percentage of the non-African ancestry.22 The same effect was observed in the present study: 17 (9.40%) of 181 subjects carrying the α-thalassemia mutation had the sickle trait, but only 22 (5.5%) of 397 without such mutations carried the sickle mutation (P = .065 one-sided Fisher exact test).
Once iron-deficient patients and patients with other causes of low TS had been excluded, the α-thalassemia gene in the African-American population accounts for about a third of the difference in hemoglobin levels between African-Americans and whites and for about one-half of the subjects classified as anemic in the African-American population. The cause of the remaining hemoglobin differences is obscure. It appears to be neither chronic renal disease nor sickle trait. Our patients were not screened for β-thalassemia but these mutations are rare among African-Americans.28 The adenosine triphosphate levels of the red cells of African-Americans are, on average, lower than those of whites,29 and the 2,3-biphosphoglycerate levels are higher.30 The latter might decrease erythropoietic drive sufficiently to result in somewhat lower hemoglobin levels. It is clear that there are multiple factors that serve to create the difference that is observed.
Medical decisions are, of necessity, based on comparing patient values with reference ranges. When these ranges are derived from one population and then applied to another, unnecessary investigations of seemingly aberrant laboratory results may be the consequence. The potential for harm from the latter type of error was recently highlighted in a study comparing African-American and white women with breast cancer. It appeared that one of the reasons why African-Americans may have a less favorable outlook is that treatment is withheld because of lower leukocyte counts.31 Decisions made by a physician regarding investigation of anemia usually are based on whether the patient's hemoglobin level falls within the accepted reference range. Our data show that even after elimination of patients with iron-deficient erythropoiesis, 16.2% of African-American women were found to have hemoglobin levels of less than 12 g/dL whereas only 3.9% of white women were in this range. A part of this difference is due to the high prevalence of α-thalassemia in the African-American population, but this is not an important practical consideration from the point of view of the physician, since means for routine clinical diagnosis of α-thalassemia are not available. The problem cannot be solved by simply establishing different ranges for different ethnic groups, especially since all represent some degree of admixture. Thus, it is basically information that the physician must possess that becomes one the many factors that we designate as clinical judgment.
Prepublished online as Blood First Edition Paper, March 24, 2005; DOI 10.1182/blood-2005-02-0713.
Supported by the National Institutes of Health grants DK53505-04 and RR00833, and the Stein Endowment Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Vincent Felitti for his helpful comments.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal