Abstract
Thrombin has been known to cause tyrosine phosphorylation of protein kinase C δ (PKCδ) in platelets, but the molecular mechanisms and function of this tyrosine phosphorylation is not known. In this study, we investigated the signaling pathways used by protease-activated receptors (PARs) to cause tyrosine phosphorylation of PKCδ and the role of this event in platelet function. PKCδ was tyrosine phosphorylated by either PAR1 or PAR4 in a concentration- and time-dependent manner in human platelets. In particular, the tyrosine 311 residue was phosphorylated downstream of PAR receptors. Also the tyrosine phosphorylation of PKCδ did not occur in Gαq-deficient mouse platelets and was inhibited in the presence of a phospholipase C (PLC) inhibitor U73122 and calcium chelator BAPTA (5,5′-dimethyl-bis(o-aminophenoxy)ethane-N, N, N ′, N ′-tetraacetic acid), suggesting a role for Gαq pathways and calcium in this event. Both PAR1 and PAR4 caused a time-dependent activation of Src (pp60c-src) tyrosine kinase and Src tyrosine kinase inhibitors completely blocked the tyrosine phosphorylation of PKCδ. Inhibition of tyrosine phosphorylation or the kinase activity of PKCδ dramatically blocked PAR-mediated thromboxane A2 generation. We conclude that thrombin causes tyrosine phosphorylation of PKCδ in a calcium- and Src-family kinase–dependent manner in platelets, with functional implications in thromboxane A2 generation.
Introduction
Platelet activation process is an important component of normal hemostasis.1-3 Secretion of granule contents from activated platelets is an important event that helps recruit more platelets to the site of injury. The importance of secretion in platelet function is emphasized by the bleeding tendencies seen in patients with storage pool disease.4-7 Multiple studies have shown that both increase in intracellular calcium and activation of protein kinase C (PKC) are required to mediate granule release.8-15
Following platelet activation, tyrosine phosphorylation events of many proteins ensue. Multiple studies have shown that these tyrosine phosphorylation events are necessary for platelet signal transduction.1,16-20 The tyrosine phosphorylation events take place in 3 waves.16 The first wave occurs following agonist-mediated activation of its receptor, independent of outside in signaling or platelet aggregation. Some of the proteins that have been shown to be tyrosine phosphorylated include Src (pp60c-src), spleen tyrosine kinase (Syk), and cortactin. This is followed by the second wave that is mediated by fibrinogen binding to the activated glycoprotein IIb and IIIa (GPIIb/IIIa).16 The third and final wave of tyrosine phosphorylation occurs as a result of the postaggregatory events that follow platelet aggregation.16
As a member of the novel PKC subfamily, PKCδ contains a carboxyl-terminal catalytic domain with 2 conserved regions, namely C3 and C4, essential for catalytic activity and substrate binding, respectively. The amino-terminal regulatory domain contains the inhibitory pseudosubstrate sequence and 2 cysteine-rich Zn-fingerlike sequences in the C1 region but lacking the calcium-binding C2 region. Following activation, PKCδ is phosphorylated at threonine and serine residues. Among these, phosphorylation of threonine 505 in the activation loop is an important event in the activation of PKCδ, and this event has been used as a marker for activation of this isoform in multiple studies.21-28 In addition to serine/threonine phosphorylations, multiple studies have shown that this isoform also gets tyrosine phosphorylated following activation by many agents, including phorbol myristate acetate (PMA), platelet-derived growth factor (PDGF), carbachol, and cholecystokinin.29-42 Previously, extensive studies have been performed to identify the tyrosine kinase that mediates the phosphorylation of tyrosine residue on PKCδ. Studies performed in other cell systems have shown that c-Src can physically associate and phosphorylate certain tyrosine residues on PKCδ.32,43-47 The role of tyrosine phosphorylation in regulating the functions of PKCδ is not completely understood and remains controversial.
Recently, we showed that PKCδ isoform plays an important role in regulating dense granule secretion in human platelets.21 The thrombin receptor-activating peptides SFLLRN and AYPGKF caused threonine phosphorylation of PKCδ, which is necessary for dense granule secretion in platelets. Use of PKCδ-selective inhibitor, Rottlerin, blocked both dense granule secretion and threonine 505 phosphorylation. Both G-protein–coupled receptor (GPCR) and glycoprotein VI (GPVI) signaling pathways lead to activation of PKCδ. However, there are significant differences in the functional role of PKCδ downstream of protease-activated receptor (PAR) and GPVI signaling in platelets as demonstrated in our previous studies.21 Platelets express 7 of the 8 members of the Src family tyrosine kinases that include Fgr (p55c-fgr), Src, Fyn (p59fyn), Lyn (p56lyn), Lck (p56lck), Hck (p59hck), and Yes (pp62c-yes).48-52 Crosby and Poole53 have shown that tyrosine phosphorylation of PKCδ in platelets can occur downstream of GPVI signaling. Even though it has been shown that thrombin can cause tyrosine phosphorylation of PKCδ in platelets,37 the exact signaling mechanism and the functional implications of this tyrosine phosphorylation event are not completely understood yet.
In this study, we show that thrombin mediates tyrosine phosphorylation of PKCδ at the tyrosine 311 residue through both PAR1 and PAR4 receptors in a calcium and Src family tyrosine kinase–dependent manner. The tyrosine phosphorylation of PKCδ appears to be important for the PAR-mediated thromboxane A2 generation but not for dense granule secretion in platelets.
Materials and methods
This study was approved by the institutional review board of Temple University, Philadelphia, PA.
Materials
Apyrase (type VII), bovine serum albumin (fraction V), thrombin, and acetylsalicylic acid were obtained from Sigma (St Louis, MO). The acetoxymethylester ester of 5,5′-dimethyl-bis-(o-aminophenoxy) ethane-N, N, N′, N′-tetraacetic acid (BAPTA), phospholipase Cβ2 (PLCβ2) inhibitor (U73122), Rottlerin, and GF109203X were from Biomol (Plymouth Meeting, PA). Hexapeptides, SFLLRN and AYPGKF, were custom synthesized at Research Genetics (Huntsville, AL). Convulxin was purchased from CenterChem (Norwalk, CT). PKCδ isoform selective antibodies, phospho-PKCδ antibodies against specific tyrosine residues, the Src family tyrosine kinase antibodies, and protein G-plus Sepharose beads were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Monoclonal phosphotyrosine antibody (clone 4G10) was purchased from Upstate Biotechnologies (Lake Placid, NY). Cell lysis buffer and phospho-PKCδ (T505) antibody were obtained from Cell Signaling Technologies (Beverly, MA).
Isolation of human platelets
All experiments using human subjects were performed in accordance with the Declaration of Helsinki. Whole blood was drawn from healthy, consenting human volunteers into tubes containing one-sixth volume of ACD (2.5 g sodium citrate, 1.5 g citric acid, and 2 g glucose in 100 mL deionized water). Blood was centrifuged (Eppendorf 5810R centrifuge, Hamburg, Germany) at 230g for 20 minutes at room temperature to obtain platelet-rich plasma (PRP). PRP was incubated with 1 mM acetylsalicylic acid for 30 minutes at 37°C. The PRP was then centrifuged for 10 minutes at 980g at room temperature to pellet the platelets. Platelets were resuspended in Tyrode buffer (138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, 0.2% bovine serum albumin) containing 0.01 U/mL apyrase. Cells were counted using the Coulter Z1 Particle Counter (Miami, FL), and concentration of cells was adjusted to 4 × 108 platelets/mL. All experiments using washed platelets were performed in the absence of extracellular calcium unless otherwise mentioned.
Isolation of mouse platelets
Blood was collected from the vena cava of anesthetized mice into syringes containing 1/10th blood volume of 3.8% sodium citrate as anticoagulant. Red blood cells were removed by centrifugation at 100g for 10 minutes. Platelet-rich plasma was recovered, and platelets were pelleted at 400g for 10 minutes. The platelet pellet was resuspended in Tyrode buffer (pH 7.4) containing 0.01 U/mL apyrase. The washed platelets were subsequently used for experiments.
Immunoprecipitation and Western blot analysis
Platelets were stimulated with agonists in the presence or absence of inhibitors for the appropriate time, and the reaction was stopped by the addition of equal volumes of the 2 × cell lysis buffer. The lysates were incubated for 30 minutes in ice for completion of the lysis. The samples were then centrifuged at 10 000g for 10 minutes in 4°C to separate the insoluble cytoskeletal elements. The supernatant was isolated, and the immunoprecipitating antibody was added in a 1:100 dilution and incubated for 2 hours in 4°C. Protein G-plus Agarose beads (40 μL) were then added, and the samples were incubated overnight with the antibody and beads at 4°C. The beads were washed 3 times with 1 × cell lysis buffer, and the proteins were eluted following addition of 3 × SDS (sodium dodecyl sulfate) Laemmlli buffer. Platelet samples were boiled for 10 minutes, and proteins were separated by 10% SDS–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membrane. Nonspecific binding sites were blocked by incubation in Tris (tris(hydroxymethyl)aminomethane)–buffered saline-Tween (TBST; 20 mM Tris, 140 mM NaCl, 0.1% (vol/vol) Tween 20) containing 2% (wt/vol) bovine serum albumin (BSA) for 30 minutes at room temperature, and membranes were incubated overnight at 4°C with the primary antibody (1:1000 dilution in TBST with 2% BSA) with gentle agitation. After 3 washes for 5 minutes each with TBST, the membranes were probed with an alkaline phosphatase-labeled secondary antibody (1:5000 dilution in TBST with 2% BSA) for 1 hour at room temperature. After additional washing steps, membranes were then incubated with chlordiazepoxide (CDP)–Star and chemiluminescent substrate (Tropix, Bedford, MA) for 10 minutes at room temperature, and immunoreactivity was detected using a Fuji Film Luminescent Image Analyzer (LAS-1000 CH; Tokyo, Japan).
Measurement of thromboxane A2 generation
Washed, human platelets without aspirin treatment were prepared and brought to a concentration of 2 × 108 platelets/mL. Stimulations were performed in a platelet aggregometer under stirring conditions (900 rpm) at 37°C. The Src family tyrosine kinase inhibitors, PP1 and PP2, and the vehicle dimethyl sulfoxide (DMSO) were added 10 minutes prior to addition of the agonist. Stimulations were performed for 3.5 minutes, and the reaction was stopped by snap-freezing. The samples were stored at –80°C until thromboxane B2 (TXB2) analysis was performed. Levels of TXB2 were determined in duplicate using a Correlate-EIA Thromboxane B2 Enzyme Immunoassay Kit (Assay Designs, Ann Arbor, MI), according to manufacturer's instructions. Data represent the average of 3 days of data plus or minus standard error.
Measurement of platelet secretion
Platelet secretion was determined by measuring the release of adenosine triphosphate (ATP) using the CHRONO-LUME (Chronolog Corp, Havertown PA) reagent. The activation of platelets was performed in a lumiaggregometer at 37°C with stirring at 900 rpm, and the secretion was measured and expressed as nmol ATP released/108 platelets. In experiments in which inhibitors were used, the platelet sample was incubated with the inhibitors for 10 minutes at 37°C prior to the addition of agonists. The secretion was subsequently measured.
Results
Role of PAR1 and PAR4 signaling in tyrosine phosphorylation of PKCδ in human platelets
Thrombin activates platelets by cleaving the G-protein–coupled receptors, PAR1 and PAR4.54 We evaluated the role of PAR1 and PAR4 activation in thrombin-mediated tyrosine phosphorylation of PKCδ in human platelets. As shown in Figure 1A, the PAR1-activating peptide, SFLLRN (50 μM), and the PAR4-activating peptide, AYPGKF (500 μM), could independently cause tyrosine phosphorylation of PKCδ. In agreement with the previous studies,37,53 both thrombin (1.0 U/mL) and glycoprotein VI (GPVI) agonist, convulxin (100 ng/mL), caused tyrosine phosphorylation of PKCδ in human platelets. The above data suggested that both PAR1 and PAR4 receptors independently contribute to thrombin-mediated tyrosine phosphorylation of PKCδ.
Effect of PAR1 and PAR4 activation on tyrosine phosphorylation of PKCδ. Washed and aspirin-treated platelets were stimulated with thrombin (1.0 U/mL), SFLLRN 50 μM, AYPGKF 500 μM, and convulxin 100 ng/mL (A), with either increasing concentrations (B-C) or varying times (D-E) of agonists as indicated at 37°C. The stimulation times for thrombin, SFLLRN, AYPGKF, and convulxin were 60, 20, 60, and 60 seconds, respectively. PKCδ was immunoprecipitated as described, and the samples were analyzed for tyrosine phosphorylation by Western blotting using the monoclonal phosphotyrosine (4G10) antibody. Equal lane loading was assured by probing the samples with PKCδ antibody. The Western blot shown is representative of experiments done using platelets from 3 different donors.
Effect of PAR1 and PAR4 activation on tyrosine phosphorylation of PKCδ. Washed and aspirin-treated platelets were stimulated with thrombin (1.0 U/mL), SFLLRN 50 μM, AYPGKF 500 μM, and convulxin 100 ng/mL (A), with either increasing concentrations (B-C) or varying times (D-E) of agonists as indicated at 37°C. The stimulation times for thrombin, SFLLRN, AYPGKF, and convulxin were 60, 20, 60, and 60 seconds, respectively. PKCδ was immunoprecipitated as described, and the samples were analyzed for tyrosine phosphorylation by Western blotting using the monoclonal phosphotyrosine (4G10) antibody. Equal lane loading was assured by probing the samples with PKCδ antibody. The Western blot shown is representative of experiments done using platelets from 3 different donors.
We also evaluated whether increasing levels of PAR1 and PAR4 activation can lead to corresponding increase in tyrosine phosphorylation of PKCδ in human platelets. As shown in Figure 1B, SFLLRN caused the tyrosine phosphorylation of PKCδ in a concentration-dependent manner, and it started at the 5-μM concentration. Similarly, AYPGKF also resulted in tyrosine phosphorylation of PKCδ in a concentration-dependent manner, which started at the 25-μM concentration (Figure 1C). These results show that both the PAR receptors can cause tyrosine phosphorylation of PKCδ in a concentration-dependent manner in human platelets.
Given the differences between the kinetics of intracellular calcium increases downstream of PAR1 and PAR4 signaling,55 we evaluated whether there exists a difference in the kinetics of tyrosine phosphorylation of PKCδ following activation of platelets with PAR1- and PAR4-activating peptides. As shown in Figure 1D, SFLLRN causes tyrosine phosphorylations of PKCδ in as early as 15 seconds and this persisted until 60 seconds. Also the maximum level of tyrosine phosphorylation was seen at 15 seconds. In contrast to SFLLRN, AYPGKF-induced tyrosine phosphorylation of PKCδ started at 15 seconds and was sustained until 120 seconds with maximum phosphorylation occurring at 60 seconds (Figure 1E). The kinetics of this tyrosine phosphorylation by PAR1 and PAR4 receptors was not altered under conditions whereby extracellular calcium (1 mM) was added and aspirin-treated platelets were used (not shown). These data suggest and confirm that there are differences in the kinetics between PAR1- and PAR4-signaling pathways, including the tyrosine phosphorylation of PKCδ mediated by activation of PAR1 and PAR4 in platelets.
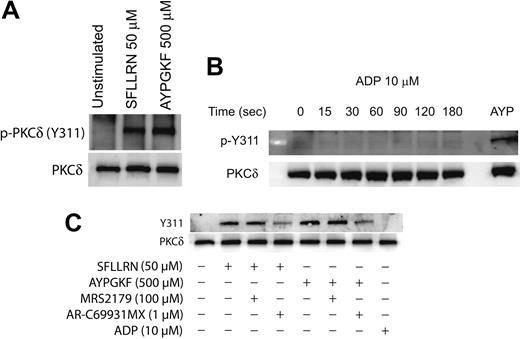
Agonist-mediated phosphorylation of Y311 of PKCδ in platelets
It is known that PKCδ can get phosphorylated on multiple tyrosine residues that includes the residues 52, 155, 187, 311, 525, and 565.34,39,45 We investigated the specific tyrosine residues that are phosphorylated downstream of the PAR signaling in platelets. This was done by stimulating platelets with the PAR-activating peptides and then measuring the phosphorylation of specific tyrosine residues by Western blotting using the different phospho-PKCδ antibodies directed against specific tyrosine residues of PKCδ. As shown in Figure 2A, only the tyrosine 311 residue was phosphorylated when platelets are activated with SFLLRN or AYPGKF. We could not detect the tyrosine phosphorylation of the other residues mentioned using the commercially available phosphospecific antibodies.
We also investigated whether ADP can cause tyrosine phosphorylation of PKCδ in platelets. Aspirin-treated washed platelets were treated with ADP (10 μM) for increasing time points, and tyrosine phosphorylation of Y311 of PKCδ was measured. As shown in Figure 2B, ADP failed to cause Y311 phosphorylation up to 3 minutes of activation. Given the important role of ADP in potentiating various platelet responses mediated by other agonists,56-60 we further wanted to evaluate the role of P2Y1 and P2Y12 receptors on the PAR-mediated tyrosine phosphorylation of PKCδ in platelets. Platelets were activated with either SFLLRN or AYPGKF in the presence and absence of the P2Y receptor antagonists and the tyrosine phosphorylation of PKCδ was measured. As shown in Figure 2C, SFLLRN and AYPGKF mediated tyrosine phosphorylation of Y311 residue of PKCδ was significantly inhibited in the presence of the P2Y12 receptor antagonist, AR-C69931MX (1 μM). In contrast, blocking the P2Y1 receptor using the antagonist, MRS2179 (100 μM) had no effect on the tyrosine phosphorylation of PKCδ. These results show that, even though ADP by itself fails to cause tyrosine phosphorylation, the P2Y12 receptor has an important role to play in the PAR-mediated tyrosine phosphorylation of PKCδ in platelets.
Agonist-mediated phosphorylation of Y311 residue of PKCδ in platelets. Washed and aspirin-treated platelets were stimulated with either SFLLRN or AYPGKF (A), 10 μM ADP (adenosine diphosphate) for different time periods (B), or with PAR agonists in the presence or absence of ADP receptor antagonists (C) at 37°C, and the reaction was stopped by adding the Laemmlli buffer. Stimulation with SFLLRN was for 20 seconds and AYPGKF was for 1 minute. The lysates were analyzed by Western blotting by using an antibody directed against the phosphorylated Y311 residue of PKCδ. Equal lane loading was assured by probing the samples with PKCδ antibody. The Western blot shown is representative of experiments done using platelets from 3 different donors. AYP indicates AYPGKF, 500 μM.
Agonist-mediated phosphorylation of Y311 residue of PKCδ in platelets. Washed and aspirin-treated platelets were stimulated with either SFLLRN or AYPGKF (A), 10 μM ADP (adenosine diphosphate) for different time periods (B), or with PAR agonists in the presence or absence of ADP receptor antagonists (C) at 37°C, and the reaction was stopped by adding the Laemmlli buffer. Stimulation with SFLLRN was for 20 seconds and AYPGKF was for 1 minute. The lysates were analyzed by Western blotting by using an antibody directed against the phosphorylated Y311 residue of PKCδ. Equal lane loading was assured by probing the samples with PKCδ antibody. The Western blot shown is representative of experiments done using platelets from 3 different donors. AYP indicates AYPGKF, 500 μM.
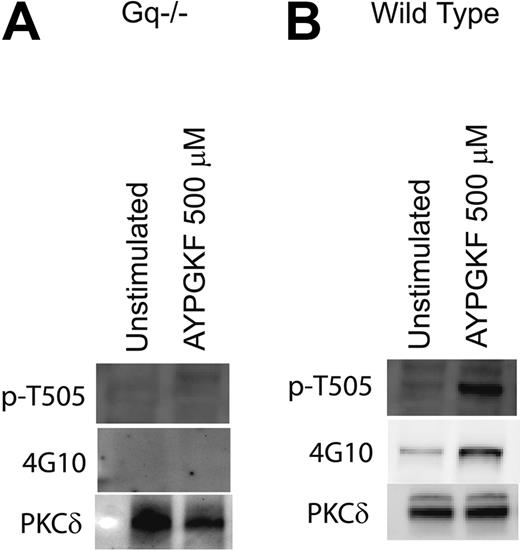
Role of Gαq in tyrosine phosphorylation of PKCδ in platelets
PAR1- and PAR4-mediated signaling in platelets occur predominantly via the activation of the Gq pathways.54 Mouse platelets deficient in the Gαq were used to evaluate the role of Gq-mediated pathways in the tyrosine phosphorylation of PKCδ. Mouse platelets express PAR4 but not PAR1.54 The PAR4-activating peptide AYPGKF was used to activate the mouse platelets, and the phosphorylation of threonine 505, which occurs downstream of Gq, was used as a positive control in these experiments. As shown in Figure 3, AYPGKF failed to cause threonine 505 and tyrosine phosphorylation of PKCδ in Gq knock-out mice (Figure 3A) in contrast to the wild-type mice platelets in which AYPGKF resulted in both threonine and tyrosine phosphorylation (Figure 3B). Similar results were obtained when the platelets were stimulated with thrombin (data not shown). This result shows and confirmed that Gq pathways are essential for PAR-mediated threonine 505 and tyrosine phosphorylation of PKCδ in platelets.
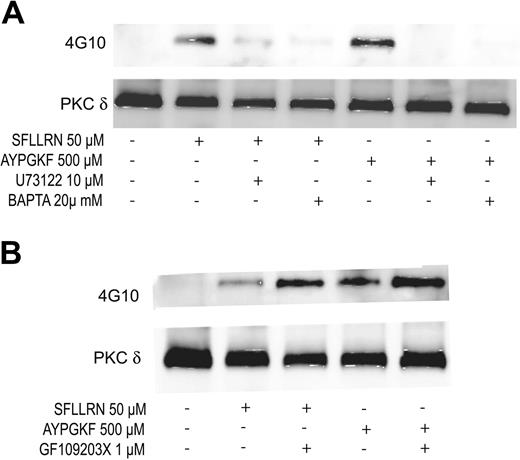
Role of phospholipase C (PLC) and downstream signaling molecules on the tyrosine phosphorylation of PKCδ
Gq-mediated PLCβ2 activation leads to the generation of IP3 (inositol 1,4,5-triphosphate) and DAG (diacylglycerol), resulting in the increase in intracellular calcium and activation of PKC. Hence, we investigated the role of PLCβ2, calcium, or PKC in the tyrosine phosphorylation of PKCδ. U73122 (PLC inhibitor), dimethyl BAPTA (calcium chelator), and GF109203X (pan-PKC inhibitor) were used to inhibit PLCβ2, calcium, and PKC, respectively. As shown in Figure 4A, both U73122 and BAPTA inhibited the SFLLRN- and AYPGKF-induced tyrosine phosphorylation of PKCδ in human platelets. In contrast, PKC inhibition by GF109203X potentiated the tyrosine phosphorylation of PKCδ downstream of PAR signaling (Figure 4B). These results suggest that PAR-mediated tyrosine phosphorylation of PKCδ is dependent on calcium that is mobilized following PLCβ2 activation.
Role of Gαq in tyrosine phosphorylation of PKCδ in platelets. Platelets from mice deficient in the Gαq protein (A) or from wild-type mice (B) were stimulated with AYPGKF (500 μM) at 37°C, and the reaction was stopped by adding the cell lysis buffer. PKCδ was immunoprecipitated as described, and the samples were analyzed for tyrosine and threonine 505 phosphorylation by Western blotting using the monoclonal phosphotyrosine (4G10) and phospho-PKCδ (T505) antibodies, respectively. Equal lane loading was assured by probing the samples with PKCδ antibody. The Western blot shown is representative of experiments done using platelets from 3 different donors.
Role of Gαq in tyrosine phosphorylation of PKCδ in platelets. Platelets from mice deficient in the Gαq protein (A) or from wild-type mice (B) were stimulated with AYPGKF (500 μM) at 37°C, and the reaction was stopped by adding the cell lysis buffer. PKCδ was immunoprecipitated as described, and the samples were analyzed for tyrosine and threonine 505 phosphorylation by Western blotting using the monoclonal phosphotyrosine (4G10) and phospho-PKCδ (T505) antibodies, respectively. Equal lane loading was assured by probing the samples with PKCδ antibody. The Western blot shown is representative of experiments done using platelets from 3 different donors.
Effect of U73122, dimethyl BAPTA, and GF109203X on the tyrosine phosphorylation of PKCδ. Aspirin-treated and washed human platelets were stimulated with SFLLRN or AYPGKF in the presence or absence of U73122 (10 μM), dimethyl BAPTA (20 μM) (A) or GF109203X (1 μM) (B) at 37°C, and the reaction was stopped by adding 2 × cell lysis buffer. DMSO was used as a vehicle control. Stimulation times for SFLLRN and AYPGKF were 20 and 60 seconds, respectively. Incubation times for U73122, dimethyl BAPTA, and GF109203X were 10, 10, and 5 minutes at 37°C, respectively. PKCδ was immunoprecipitated as described, and the samples were analyzed for tyrosine phosphorylation by Western blotting using the monoclonal phosphotyrosine (4G10) antibody. Equal lane loading was assured by probing the samples with PKCδ antibody. The Western blot shown is representative of experiments done using platelets from 3 different donors.
Effect of U73122, dimethyl BAPTA, and GF109203X on the tyrosine phosphorylation of PKCδ. Aspirin-treated and washed human platelets were stimulated with SFLLRN or AYPGKF in the presence or absence of U73122 (10 μM), dimethyl BAPTA (20 μM) (A) or GF109203X (1 μM) (B) at 37°C, and the reaction was stopped by adding 2 × cell lysis buffer. DMSO was used as a vehicle control. Stimulation times for SFLLRN and AYPGKF were 20 and 60 seconds, respectively. Incubation times for U73122, dimethyl BAPTA, and GF109203X were 10, 10, and 5 minutes at 37°C, respectively. PKCδ was immunoprecipitated as described, and the samples were analyzed for tyrosine phosphorylation by Western blotting using the monoclonal phosphotyrosine (4G10) antibody. Equal lane loading was assured by probing the samples with PKCδ antibody. The Western blot shown is representative of experiments done using platelets from 3 different donors.
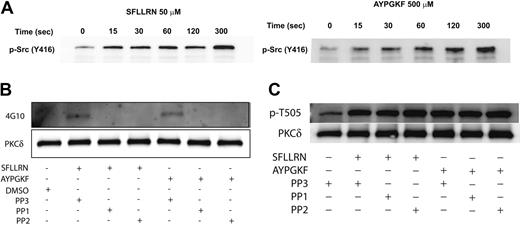
Effect of Src-family tyrosine-kinase inhibition on PAR-mediated threonine and tyrosine phosphorylation of PKCδ in platelets
Since the Src family tyrosine kinases are the predominant tyrosine kinases found in platelets,61 we investigated whether the Src family tyrosine kinases are phosphorylated and activated downstream of the PAR peptides in platelets and further whether these kinases were involved in tyrosine phosphorylation of PKCδ. Platelets have been shown to contain several members of the Src family tyrosine kinase that include Fgr, Fyn, Lck, Lyn, Src, Hck, and Yes.51,52,61 The platelets were stimulated with either SFLLRN or AYPGKF for the different time points as indicated, and the activation of the Src tyrosine kinases was measured by Western blotting using the phospho-Src (Y416) antibody. As shown in Figure 5A, there was a time-dependent phosphorylation and activation of the Src tyrosine kinase downstream of the PAR-activating peptide signaling. To determine whether the Src family tyrosine kinases are involved in the tyrosine phosphorylation of PKCδ, we measured the tyrosine phosphorylation of PKCδ in the presence and absence of the Src family inhibitors, PP1 (10 μM) and PP2 (10 μM). The structural analog PP3 (10 μM), with no Src-inhibiting activity, was used as a negative control. As shown in Figure 5B, the tyrosine phosphorylation of PKCδ mediated by either SFLLRN or AYPGKF was completely abolished by both PP1 and PP2. However, the negative structural control, PP3, had no effect on the tyrosine phosphorylation mediated by these agonists. In contrast, blocking the Src family tyrosine kinases using either PP1 or PP2 had absolutely no effect on the threonine 505 phosphorylation of PKCδ (Figure 5C). From the above results, it is evident that the Src family of tyrosine kinases are activated by the PAR agonists and that these are the upstream kinases that are involved in the tyrosine phosphorylation (but not threonine 505) of PKCδ in platelets.
Effect of inhibitors Rottlerin and PP2 on thromboxane-A2 generation and dense-granule secretion in human platelets
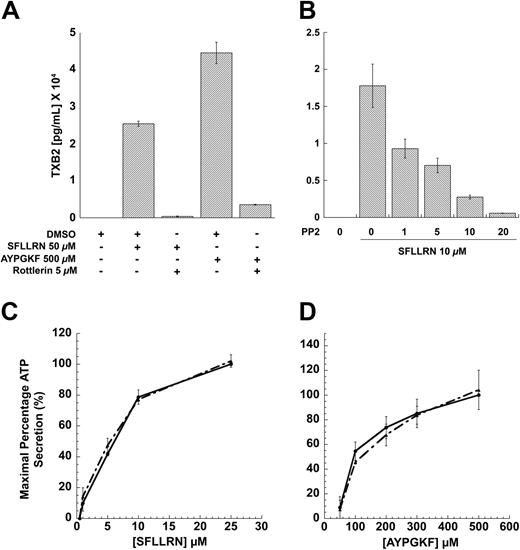
Earlier studies have shown that PKCδ is important in mediating the dense-granule secretion21 and thromboxane A2 generation downstream of PAR signaling.62 It is well known that the threonine-505 residue in the catalytic loop of PKCδ is important for its activity and, hence, dense-granule secretion.21 Inhibition of this phosphorylation results in blockade of dense-granule secretion. We investigated the role of PKCδ in PAR-mediated thromboxane-A2 generation, and our results show that both SFLLRN- and AYPGKF-mediated thromboxane-A2 generation is dramatically inhibited in the presence of the PKCδ-selective inhibitor, Rottlerin (Figure 6A). These data showed and confirmed the finding that PKCδ plays an important role in PAR-mediated thromboxane-A2 generation in human platelets.
The activation of Src family tyrosine kinases downstream of PAR stimulation in platelets. Washed and aspirin-treated platelets were stimulated with either SFLLRN (50 μM) or AYPGKF (500 μM) for the different time points at 37°C, and the activation of Src family tyrosine kinases were studied by Western blotting using the phospho-Src (Y416) antibody (A). Washed and aspirin-treated platelets were stimulated with either SFLLRN or AYPGKF in the presence and absence of PP1 (10 μM) or PP2 (10 μM) at 37°C, and the tyrosine phosphorylation of PKCδ was measured (B). PP3 (10 μM) was used as a negative control. Washed and aspirin-treated platelets were stimulated with either SFLLRN or AYPGKF in the presence or absence of 10 μM PP1 or PP2, and the threonine 505 phosphorylation was measured by Western blotting using the phospho-PKCδ (T505) antibody (C). The Western blots shown are representative of experiments performed using 3 different donors.
The activation of Src family tyrosine kinases downstream of PAR stimulation in platelets. Washed and aspirin-treated platelets were stimulated with either SFLLRN (50 μM) or AYPGKF (500 μM) for the different time points at 37°C, and the activation of Src family tyrosine kinases were studied by Western blotting using the phospho-Src (Y416) antibody (A). Washed and aspirin-treated platelets were stimulated with either SFLLRN or AYPGKF in the presence and absence of PP1 (10 μM) or PP2 (10 μM) at 37°C, and the tyrosine phosphorylation of PKCδ was measured (B). PP3 (10 μM) was used as a negative control. Washed and aspirin-treated platelets were stimulated with either SFLLRN or AYPGKF in the presence or absence of 10 μM PP1 or PP2, and the threonine 505 phosphorylation was measured by Western blotting using the phospho-PKCδ (T505) antibody (C). The Western blots shown are representative of experiments performed using 3 different donors.
Given that PAR agonists can also cause tyrosine phosphorylation, we investigated whether this phosphorylation event is important for dense granule secretion, thromboxane A2 generation, or both. We addressed the question by measuring the PAR-mediated dense granule secretion and thromboxane A2 generation under conditions whereby the tyrosine phosphorylation of PKCδ is blocked (by using PP2). As shown in the Figure 6B, PP2 concentration dependently blocked the thromboxane A2 generation occurring downstream of PAR1 signaling. Similar results were also obtained for AYPGKF-induced thromboxane A2 generation. In contrast, blocking the Src family tyrosine kinases using PP2 had no effect on the dense granule secretion mediated by SFLLRN (Figure 6C) or AYPGKF (Figure 6D). From these results, we conclude that the tyrosine phosphorylation of PKCδ might be important for the PAR-mediated thromboxane A2 generation, but not dense granule secretion. In contrast, the threonine phosphorylation seems to be important for both dense granule secretion and thromboxane A2 generation mediated by PAR signaling in platelets (Figure 7).
Effect of inhibitors on PAR-mediated thromboxane A2 generation and dense granule release in human platelets. Washed platelets without aspirin treatment were stimulated with SFLLRN or AYPGKF in the presence or absence of 5μM Rottlerin (A) or with SFLLRN in the presence of different concentrations of PP2 (B) at 37°C, and the generated thromboxane B2 was measured as described in “Results” by ELISA. Washed and aspirin-treated human platelets were stimulated with different concentrations of SFLLRN (C) or AYPGKF (D) in the presence of 10 μM PP2 (broken lines) or 10 μM PP3 (solid lines), and the dense granule secretion was measured using Lumichrome reagent. The mean ± SD is derived from experiments performed using platelets obtained from 3 independent donors.
Effect of inhibitors on PAR-mediated thromboxane A2 generation and dense granule release in human platelets. Washed platelets without aspirin treatment were stimulated with SFLLRN or AYPGKF in the presence or absence of 5μM Rottlerin (A) or with SFLLRN in the presence of different concentrations of PP2 (B) at 37°C, and the generated thromboxane B2 was measured as described in “Results” by ELISA. Washed and aspirin-treated human platelets were stimulated with different concentrations of SFLLRN (C) or AYPGKF (D) in the presence of 10 μM PP2 (broken lines) or 10 μM PP3 (solid lines), and the dense granule secretion was measured using Lumichrome reagent. The mean ± SD is derived from experiments performed using platelets obtained from 3 independent donors.
Discussion
GPCR and GPVI signaling are 2 dominant signaling pathways that mediate many of the important functional responses in platelets. Collagen and thrombin are 2 important physiologic agonists that signal through GPVI and GPCR pathways, respectively. Platelet dense granule secretion requires both an increase in the intracellular calcium and the activation of PKC. Recently, our laboratory and others showed that PKCδ is important in regulating dense granule secretion in human platelets.21,53 In addition to the serine/threonine phosphorylations, PKCδ has been demonstrated to be tyrosine phosphorylated as well in response to various stimuli in different cell systems.29-42 Even though thrombin has been shown to tyrosine phosphorylate PKCδ in human platelets, the exact signaling mechanism by which this occurs has not been studied.37 Given the importance of thrombin signaling in platelets and the role of PKCδ in regulating dense granule secretion, it is important to understand the molecular mechanism and the functional implications of tyrosine phosphorylation of PKCδ isoform downstream of thrombin activation in platelets.
Thrombin mediates its action on platelets through the protease-activated receptors PAR1 and PAR4.54 In cultured myocytes, PAR4 peptide AYPGKF but not PAR1 peptide SFLLRN caused Src activation.63 Given this difference in signaling between the 2 PAR receptors, we investigated the role of PAR1 and PAR4 receptors in thrombin-mediated tyrosine phosphorylation of PKCδ. Our results indicate that the tyrosine phosphorylation of PKCδ is an event that occurred as a function of one or more tyrosine kinase that is downstream of both PAR1 and PAR4 activation in platelets. Our results also show that activation of PAR1 and PAR4 receptors results in phosphorylation of Y311 alone, and none of the other tyrosine residues are phosphorylated under these conditions.
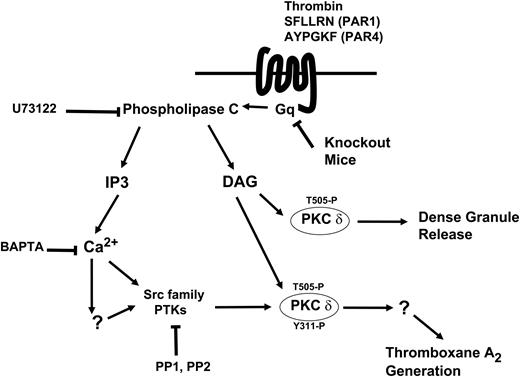
Model depicting the mechanism and the function of tyrosine phosphorylation of PKCδ following PAR stimulation in human platelets. Thrombin acts through PAR1 and PAR4 receptors and causes activation of the Gq/PLC pathways. PLC activation leads to generation of IP3, which mobilizes intracellular calcium. Increase in calcium directly or indirectly leads to increase in Src activity and subsequently tyrosine phosphorylation of PKCδ. DAG leads to phosphorylation of the threonine 505 residues, which is required for the dense granule secretion. Tyrosine phosphorylation of the Y311 residue mediated through the Src family PTKs is required for thromboxane A2 generation downstream of PAR agonists.
Model depicting the mechanism and the function of tyrosine phosphorylation of PKCδ following PAR stimulation in human platelets. Thrombin acts through PAR1 and PAR4 receptors and causes activation of the Gq/PLC pathways. PLC activation leads to generation of IP3, which mobilizes intracellular calcium. Increase in calcium directly or indirectly leads to increase in Src activity and subsequently tyrosine phosphorylation of PKCδ. DAG leads to phosphorylation of the threonine 505 residues, which is required for the dense granule secretion. Tyrosine phosphorylation of the Y311 residue mediated through the Src family PTKs is required for thromboxane A2 generation downstream of PAR agonists.
Signaling downstream of the P2Y12 receptors have been identified to be important for the platelet responses mediated by other agonists like thrombin, collagen, and thromboxane A2.56-60 In agreement with these studies we also found that signaling by the P2Y12 receptor is important for the tyrosine phosphorylation of PKCδ in platelets. Blocking the P2Y12 receptor using AR-C69331MX significantly inhibited the tyrosine phosphorylation of PKCδ (Figure 2C). ADP activation of platelets by itself fails to cause tyrosine phosphorylation of PKCδ (Figure 2B). The precise mechanism through which P2Y12 receptor signaling mediates this effect is speculative at this moment. It could be that it potentiates the amount of intracellular calcium mobilized by the PAR agonists or maybe there is enhanced activation of Src family tyrosine kinases downstream of P2Y12 receptor that contributes to the PAR-mediated tyrosine phosphorylation of PKCδ. A recent study from our laboratory has shown that Src family tyrosine kinases are activated downstream of P2Y12 signaling in platelets.64 Even though the identity of the specific member of the Src family kinases downstream of P2Y12 receptor is not known at this point, some of these members might contribute to the tyrosine phosphorylation of PKCδ mediated by the PAR agonists in platelets.
As the Gαq-mediated signaling pathways are the main events that occur following PAR1 and PAR4 receptor activation, we evaluated the role of Gαq and its downstream effectors in the tyrosine phosphorylation of PKCδ. Our data indicate that the tyrosine phosphorylation of PKCδ depends on Gαq, PLC, and calcium pathways. Differences in the kinetics of activation and desensitization between PAR1 and PAR4 in their sensitivity to activation by thrombin have been well documented.65 Activation of platelets with PAR1 peptide, SFLLRN, induces a rapid calcium spike in contrast to the slow and sustained calcium response as is seen when platelets are activated with PAR4 peptide, AYPGKF.55 Similar to calcium mobilization, the kinetics of dense granule secretion and PKCδ activation are different between the 2 PAR receptor-mediated signaling pathways in platelets.21 In agreement with the above-mentioned studies, the tyrosine phosphorylation of PKCδ also followed a kinetic pattern characteristic of PAR1 and PAR4 signaling (Figure 1D-E). The pattern of calcium mobilization seen when platelets are activated with SFLLRN is very similar to the pattern of tyrosine phosphorylation of PKCδ. Similarly, the pattern of slow and sustained calcium mobilization seen with AYPGKF is similar to the sustained tyrosine phosphorylation of PKCδ seen in platelets. All these studies and observations are in favor of an important role for intracellular calcium increases in the tyrosine phosphorylation of PKCδ. However, it should be noted that the phosphorylation of Thr505 and the activation of PKCδ occur in a calcium-independent manner.
Interestingly though, inhibition of PKC using a pan-PKC inhibitor potentiated the tyrosine phosphorylation of PKCδ (Figure 4B). This result was not anticipated but could probably be due to the inhibition of certain PKC isoforms that might play a negative role in regulating the tyrosine kinase responsible for tyrosine phosphorylation of PKCδ. There have been studies showing the interaction between PKC and tyrosine kinases such as the Src family tyrosine kinase whereby each of these kinases phosphorylate and regulate the activity of the other.53,66-70 Such similar results were observed in a recent study in which the tyrosine phosphorylation of PKCα was potentiated when the activity of PKC isoforms were blocked by a broad PKC inhibitor.71
Platelets express different nonreceptor tyrosine kinases with the Src family of tyrosine kinases being the predominant one. Since studies have shown the involvement of the Src family of tyrosine kinases in the regulation of PKCδ, we investigated whether these tyrosine kinases are activated downstream of the PAR signaling. The availability of an antibody directed against the phosphorylated Y416 residue in the Src kinases has enabled us to study the activation of these kinases following platelet activation. As our results show in Figure 5A, both PAR1 and PAR4 signaling leads to significant phosphorylation of the Y416 residues, suggesting that there is Src activation occurring downstream of this signaling pathway in platelets. On the basis of the results from the Western blotting, we hypothesize that c-Src or Fyn might be involved in this tyrosine phosphorylation of PKCδ. Furthermore, a previous study has shown that Fyn is involved in the tyrosine phosphorylation of PKCδ downstream of GPVI signaling.53
Downstream of PAR stimulation, blocking PLC activation leads to inhibition of threonine and tyrosine phosphorylation of PKCδ, while BAPTA blocks only the tyrosine phosphorylation but not threonine phosphorylation. This suggests that there maybe a Src tyrosine kinase that is calcium dependent, which is important in causing the tyrosine phosphorylation of PKCδ. Also more recently, the classical calcium-dependent PKCα isoform was shown to associate with the tyrosine kinases, Src and Syk, suggesting a role for calcium in activation of tyrosine kinase.71 But the question “do the activated Src phosphorylate PKCδ?” was addressed using the Src family inhibitors PP1 and PP2. As shown in Figure 5B, inhibition of these Src family tyrosine kinases, using PP1 and PP2, completely abolished the tyrosine phosphorylation of PKCδ. Use of the pan-Src family kinase inhibitors, PP1 and PP2, does not distinguish the specific member(s) of this tyrosine kinase family that causes the tyrosine phosphorylation of PKCδ. Lack of inhibitors selective for each of the Src family tyrosine kinases makes it difficult to define the identity of the specific upstream kinase.
What is the possible role of this tyrosine phosphorylation of PKCδ? We have established the role of threonine 505 phosphorylation and activation of PKCδ in dense granule secretion. We investigated whether the Src family tyrosine kinases regulate the threonine phosphorylation and dense granule secretion. In our hands, inhibition of Src family tyrosine kinases (using PP1 or PP2) had no effect on the threonine phosphorylation of PKCδ (Figure 5C). We also found that blocking the Src family tyrosine kinases using PP2 had no effect on the dense granule secretion mediated by the PAR agonists (Figure 6C-D) Thus, the tyrosine phosphorylation is not required for the PAR-mediated dense granule release in platelets.
We found that inhibiting PKCδ with Rottlerin significantly inhibited the PAR-mediated thromboxane A2 generation. It can be seen that PAR4-mediated TXA2 generation is almost twice as much as that generated following PAR1 activation. It is likely that phospholipase A2 (PLA2) activation, a requirement for TXA2 generation, could be regulated differently downstream of PAR1 and PAR4 signaling. The difference in the receptor kinetics that exists between PAR1 and PAR4 with regard to calcium mobilization could account for the difference in the thromboxane levels that are generated by PAR1 and PAR4 activation in platelets. Thus, the sustained intracellular calcium levels upon PAR4 stimulation might contribute to enhanced thromboxane generation. Our results show that inhibition of Src family tyrosine kinase blocked both the tyrosine phosphorylation and thromboxane A2 generation mediated by the PAR agonists, indicating that tyrosine phosphorylated PKCδ might initiate signaling events distinct from the nontyrosine phosphorylated PKCδ and that the signaling events initiated by the tyrosine phosphorylated PKCδ might be essential for thromboxane A2 generation.
In conclusion, thrombin-mediated tyrosine phosphorylation of PKCδ can result from either PAR1 or PAR4 receptor activation. This phosphorylation is dependent on calcium and Src family tyrosine kinases. Finally, tyrosine 311 phosphorylation of PKCδ is not required for PAR-mediated dense granule release, but it is essential for thromboxane A2 generation by PAR agonists.
Prepublished online as Blood First Edition Paper, April 5, 2005; DOI 10.1182/blood-2004-12-4866.
Supported by research grants from the National Institutes of Health (HL60683 and HL80444) (S.P.K.) and predoctoral fellowships from the American Heart Association, Pennsylvania-Delaware affiliate (S.M. and H.S.).
S.M. wrote the paper, designed the experiments, performed the research, and analyzed the data; H.S., R.T.D., and J.J. designed and performed the experiments and analyzed the data; S.B. performed the experiments; and S.P.K. designed the experiments, analyzed the data, and provided overall direction.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal