Abstract
Hematopoiesis involves the production of stem cells, followed by the orchestrated differentiation of the blood lineages. Genetic screens in zebrafish have identified mutants with defects that disrupt specific stages of hematopoiesis and vasculogenesis, including the cloche, spadetail (tbx16), moonshine (tif1g), bloodless, and vlad tepes (gata1) mutants. To better characterize the blood program, gene expression profiling was carried out in these mutants and in scl-morphants (sclmo). Distinct gene clusters were demarcated by stage-specific and mutant-specific gene regulation. These were found to correlate with the transcriptional program of hematopoietic progenitor cells, as well as of the erythroid, myeloid, and vascular lineages. Among these, several novel hematopoietic and vascular genes were detected, for instance, the erythroid transcription factors znfl2 and ncoa4. A specific regulation was found for myeloid genes, as they were more strongly expressed in vlt mutants compared with other erythroid mutants. A unique gene expression pattern of up-regulated isoprenoid synthesis genes was found in cloche and sclmo, possibly in migrating cells. In conjunction with the high conservation of vertebrate hematopoiesis, the comparison of transcriptional profiles in zebrafish blood mutants represents a versatile and powerful tool to elucidate the genetic regulation of blood and blood vessel development.
Introduction
Vertebrate hematopoiesis is a highly conserved genetic program that defines the differentiation of hematopoietic stem cells (HSCs) into mature blood cells of all lineages (reviewed in Galloway and Zon1 ). In mammals, as well as in zebrafish, hematopoiesis occurs in 2 sequential and spatially distinct waves. The transient, primitive wave originates from ventral mesoderm and gives rise to primitive erythrocytes and macrophages. In zebrafish, primitive hematopoietic progenitors initially reside in bilateral stripes of posterior lateral plate mesoderm, and subsequently converge to the midline, forming the intermediate cell mass (ICM). The ICM represents the equivalent of the extraembryonic mammalian yolk sac blood island and gives rise to primitive erythroid and endothelial precursors. A more anterior site, called the rostral blood island (RBI), is responsible for the formation of primitive macrophages and granulocytes.2-4 Although precursors in both the ICM and RBI are specified at approximately the 3-somite stage, primitive blood cells do not enter circulation until the onset of heart contractions at 26 to 28 hours postfertilization (hpf).5 The second and definitive hematopoietic wave begins around 30 hpf, in which blood cells of the erythroid, myeloid, and lymphoid lineages are thought to arise from self-renewing HSCs that reside in the ventral wall of the dorsal aorta.6-8 This site corresponds to the aorta-gonad-mesonephros (AGM) region of other vertebrates (reviewed in Yoder and Palis9 ).
The hematopoietic waves are regulated by transcription factors that are highly conserved among vertebrates. In zebrafish, several blood mutants have been identified from 2 large-scale forward genetic screens. These mutants exhibit various hematopoietic defects ranging from impaired blood stem cell differentiation during early somitogenesis10 to defects in erythrocyte maturation.11-13 A high number of these defects are caused by genetic mutations of hematopoietic transcription factors (reviewed in Wingert and Zon14 ). The vlad tepes (vlt) mutant is characterized by an absence of the erythroid cell lineage, caused by a defect in the erythroid transcription factor gata1.15 Similarly to vlt, embryos homozygous for the moonshine (mon) mutation encoding tif1g also display an erythropoietic defect.16 The bloodless (bls) mutants lack erythroid cells during the primitive wave of hematopoiesis; however, functional definitive blood cells arise at later developmental stages. The still-unidentified bls gene is therefore considered to act specifically on primitive blood progenitors.17 Disruption of T-box gene 16 (tbx16), the gene underlying the defect in spadetail (spt) mutants, results in disorganized trunk somites and improper differentiation of ICM and AGM blood precursors.18 The spt mutants show specification of myeloid and monocytic precursors from the RBI and of angioblasts,8,19 suggesting that spt embryos have a specific block in posterior hematopoietic progenitor formation.8,20
The cloche (clo) mutants display hematopoietic and vascular defects.21 The clo gene is therefore considered to act at the level of the putative hemangioblast that gives rise to both blood and blood vessel progenitors.7,8,22,23 Overexpression of scl, a gene that is critical for the formation of HSCs, can rescue the hematopoietic defect in clo,7,24 suggesting that the clo gene acts upstream of scl. Consistent with these findings, wild-type (WT) embryos injected with a morpholino against scl (sclmo) exhibit a phenotype that is similar to clo mutants, with defects in hematopoietic differentiation and, at later stages, in vascular remodeling. Primary blood vessel formation is unaffected in sclmo embryos, and vascular progenitor markers, such as flk1, are normally expressed at early developmental stages.25 The availability of such mutants provides a means to dissect the function of the genes required for blood cell development during vertebrate ontogeny.
In this study, we determined the gene expression profile for each of these mutants. We demonstrate that similar and differential gene expression between clo, spt, mon, vlt, bls, and sclmo outlines the stage- and lineage-specific regulation of the hematopoietic genetic program, since we detect early critical transcription factors, such as scl, lmo2, and gata1, as well as mature blood cell markers of the erythroid, myeloid, and vascular lineages. Several novel hematopoietic and vascular genes were identified. In addition, a novel expression phenotype in clo mutants and in sclmo was revealed, characterized by the increased expression of isoprenoid synthesis genes. Taken together, our study demonstrates that microarray analysis of zebrafish blood mutants can provide new insights into hematopoietic and vascular development.
Materials and methods
Zebrafish husbandry
Zebrafish were raised and maintained in The Children's Hospital (Boston, MA) Zebrafish Facility according to established techniques.26 Embryos were obtained by pairwise matings of adult Tu, AB, or TL fish, raised at 28°C, and staged according to Kimmel et al.27 Zebrafish embryos at the 14-somite stage (clom39/lmo2-GFP,28 scl-morphants25 ) and at the 36 hpf stage (clom39 [deletion allele, clos5 [point mutation allele], montb222, and montg234, vltm651, sptb104, blsh75, sclmo) were collected from 3 clutches each, with the exception of clos5 (4 clutches) and vltm651 (2 clutches). In each clutch, mutant and WT siblings were separated and processed in parallel.
RNA extraction
After homogenization (Tekmar Tissumizer, Cincinnati, OH), total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA), and purified on RNeasy resins (Qiagen, Valencia, CA) according to the manufacturer's recommendations. The quantity and quality of total RNA was assessed by absorbance at 260 nm and 280 nm and by gel electrophoresis.
Target preparation, hybridization, and signal detection
Total RNA (7 μg) was converted to complementary DNA (cDNA) (Superscript II kit; Invitrogen) by priming with an oligo(dT) primer that included a T7 RNA polymerase promoter site at the 5′ end. cDNA was then used directly in an in vitro transcription reaction in the presence of biotinylated nucleotides (BioArray High Yield RNA Transcript Labeling Kit; Enzo, Farmingdale, NY) to produce biotin-labeled cRNA (antisense RNA). cRNA (15 μg) was subsequently fragmented (Ambion, Austin, TX) and hybridized to Affymetrix zebrafish GeneChips (Affymetrix, Santa Clara, CA; http:www.affymetrix.com/products/arrays/specific/zebrafish.affx),29 according to the manufacturer's guidelines. After staining with a streptavidin-phycoerythrin conjugate (Molecular Probes, Eugene, OR), the fluorescence of bound RNA was quantified using a GeneChip scanner (Affymetrix).
Method for identifying differentially expressed genes, based on a control spike-in dataset
We chose to apply a set of Affymetrix analysis methods that optimize the detection of differentially expressed genes, according to a recently developed control spike-in dataset.30 Most of these methods are implemented in Bioconductor's “affy” package,31 supplemented with available custom scripts (http://www.elwood9.net/spike); for details, refer to Choe et al.30 Briefly, the procedure involves the generation of 8 expression summary datasets, using the Microarray Suite software (MAS; Affymetrix) background correction method,32,33 4 different normalization algorithms (loess, invariant set, quantile, and constant), the MAS 5.0 estimate of nonspecific signal,32 and 2 different robust estimators (Tukey-Biweight and median polish). The 8 expression summary datasets are then combined to generate a summary significance statistic that includes an intensity-dependent error model, derived from CyberT (http://visitor.ics.uci.edu/genex/cybert/).34 Permutation testing results in false discovery rate estimates corresponding to various significance statistic levels. For details, refer to Choe et al.30
The probe sets on the chip were initially filtered for genes more likely to be involved in differential expression; these filters were (1) fold change more than 1.25 or less than 0.8 in at least one genotype-to-wild-type (WT) comparison, (2) presence under at least one condition using the MAS 5.0 absent-present call,32 and (3) q value equal to 0.15 in at least one clo-to-WT comparison.
Agglomerative (hierarchical) cluster analysis was performed on the log fold-change values for the filtered probe sets, using centroid linkage and an uncentered Pearson correlation coefficient as the distance metric.35 Ambiguities in the ordering of nodes were resolved by using the results of a self-organizing map (SOM, employing an uncentered correlation coefficient). Clustergrams were visualized using publicly available software.36
Gene annotation
Affymetrix probe sets were annotated based on gene symbols available from Affymetrix or UniGene (build no. 78)37 or by similarity of the corresponding zebrafish genomic sequences38 to human proteins.39 For Affymetrix probe sets with no significant similarity to mammalian proteins, the Affymetrix probe set ID is given.
Whole-mount in situ hybridization
cDNA clones (Open Biosystems, AL) corresponding to Affymetrix consensus sequences (http:www.affymetrix.com/products/arrays/specific/zebrafish.affx) were linearized, and digoxigenin-labeled RNA probes were transcribed according to the manufacturer's guidelines (Roche Molecular Biochemicals, Indianapolis, IN). Whole-mount in situ hybridization was performed as previously described40 with a few modifications. Embryos that had developed melanocytes were incubated in a bleach solution (0.8% KOH, 0.9% H2O2, 0.1% Tween-20 in deionized H2O) until pigment was no longer visible, washed in PBST (1X phosphate-buffered saline [PBS]; 0.1% Tween-20) and fixed in 4% paraformaldehyde/PBST. Visible light imaging was performed using a Leica MZ16 microscope (Leica, Northvale, NJ) with 60- to 100-fold magnification and a Nikon E995 digital camera (Nikon, Melville, NY). Digital images were further processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Results
Blood cell development is regulated by combinations of critical transcription factors that lead to differentiation. Several zebrafish mutants have distinct defects in blood development, and their analysis helps to elucidate the role of these transcription factors in vertebrate hematopoiesis. To determine the transcriptional profile of these mutants during blood formation, gene expression analysis was performed in the mutants and their WT siblings. We used Affymetrix zebrafish GeneChips to allow the simultaneous expression analysis of approximately 14 900 zebrafish transcripts (http:www.affymetrix.com/products/arrays/specific/zebrafish.affx). Embryos were examined at 14 somites and at 36 hpf, 2 developmental stages during which genes of the primitive wave are considered to be expressed. As gene expression of the second wave is believed to initiate around 30 hpf,6-8 the later timepoint examined also the early stages of the definitive hematopoiesis. The phenotype of cloche is not visible in live embryos until approximately 32 hpf. Therefore, to identify mutants at the 14-somite stage, we took advantage of transgenic zebrafish expressing green fluorescent protein (GFP) under the control of the lmo2 promotor (lmo2-GFP).28 Expression of the transgene was very low in transgenic clom39lmo2-GFP embryos, but abundant in the ICM of their WT siblings (data not shown). At 36 hpf, clom39 (deletion), clos5 (point mutation), spt, mon, vlt, and bls mutants were identified based on reduced numbers of circulating blood cells with respect to their WT siblings. sclmo embryos25 were collected at the 14-somite stage and at 36 hpf and compared with their uninjected siblings.
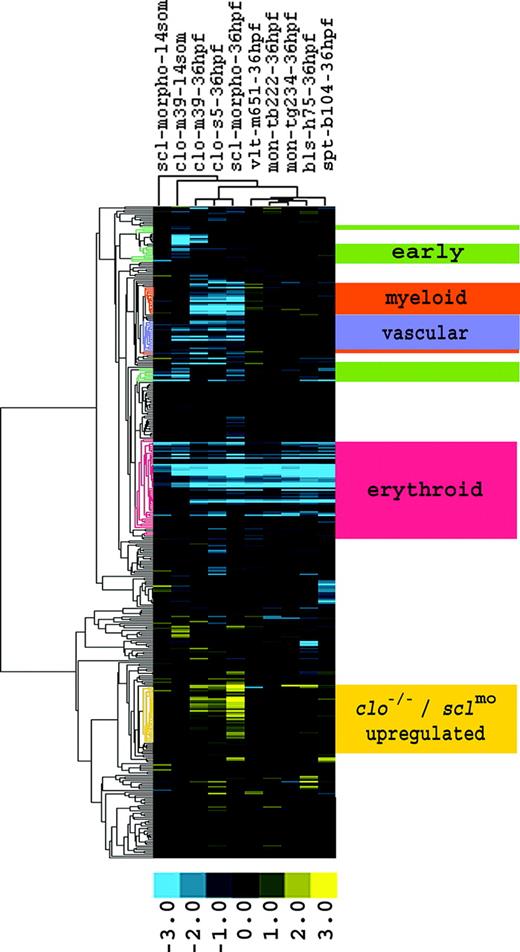
Comparison of gene expression profiles demarcates clusters with similar and differential gene regulation between mutants. There were 387 genes differentially expressed between WT and clo embryos. The expression responses of these genes were then determined in clo, vlt, mon, bls, and spt mutants, as well as in sclmo, and hierarchically clustered. Four clusters that matched the predicted expression profiles of the early hematopoietic and vascular genes, of the erythroid, myeloid, and vascular lineages, and an additional cluster with genes up-regulated in clo and sclmo embryos, are highlighted. Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos.
Comparison of gene expression profiles demarcates clusters with similar and differential gene regulation between mutants. There were 387 genes differentially expressed between WT and clo embryos. The expression responses of these genes were then determined in clo, vlt, mon, bls, and spt mutants, as well as in sclmo, and hierarchically clustered. Four clusters that matched the predicted expression profiles of the early hematopoietic and vascular genes, of the erythroid, myeloid, and vascular lineages, and an additional cluster with genes up-regulated in clo and sclmo embryos, are highlighted. Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos.
We based our analysis on the set of genes that was differentially regulated in clo mutants. Three individual comparisons between clo embryos (clom39 at 14 somites and at 36 hpf, and clos5 at 36 hpf) and their respective WT siblings were combined, resulting in 387 genes with significantly regulated expression in at least 1 of 3 clo samples (Supplemental Table S1; see the Supplemental Materials link at the top of the online article on the Blood website). We then observed the expression pattern of these 387 genes in other hematopoietic mutants, including vlt, mon, bls, and spt, as well as sclmo. This comparison revealed the differential expression of these genes between the mutants and between developmental stages. Hierarchical clustering according to the regulation of the 387 genes in each mutant and WT pair demarcated 5 gene clusters with distinct expression patterns (Figure 1). Based on the phenotypes of the mutants, these clusters were assigned to early regulatory factors, to the erythroid, myeloid, and vascular cell lineages, as well as to genes up-regulated in clo mutants and sclmo embryos.
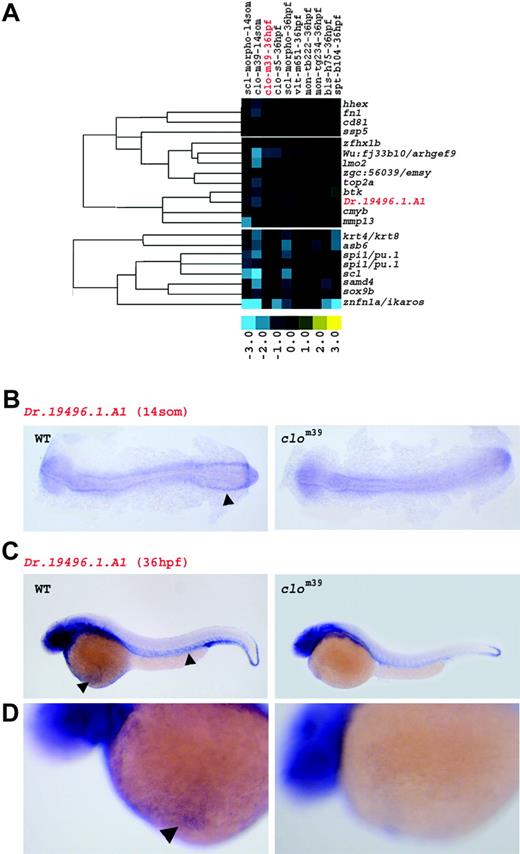
Stronger down-regulation in clo at 14 somites than at 36 hpf reveals early hematopoietic and vascular genes. (A) Genes in this cluster are marked by a predominant decrease in expression in clom39 at 14 somites (som). Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos. The expression of genes highlighted in red is illustrated by in situ hybridization in panels B, C, and D. (B) At 14 somites, transcripts of Dr.194 96.1.A1 are present in the ICM of WT (arrowhead), but not in clom39. (C) At 36 hpf, expression of Dr.194 96.1.A1 is weakly detected in vasculature and in circulating erythrocytes (arrowheads) of WT embryos, but almost completely absent in clom39. Additional expression of Dr.194 96.1.A1 in epidermal cells, in the brain, and in the eye is present in both WT and clom39. (D) Higher magnification of panel C. Transcripts of Dr.194 96.1.A1 are detected in circulating erythrocytes (arrowhead) on the yolk sac of WT, but not of clom39 embryos at 36 hpf.
Stronger down-regulation in clo at 14 somites than at 36 hpf reveals early hematopoietic and vascular genes. (A) Genes in this cluster are marked by a predominant decrease in expression in clom39 at 14 somites (som). Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos. The expression of genes highlighted in red is illustrated by in situ hybridization in panels B, C, and D. (B) At 14 somites, transcripts of Dr.194 96.1.A1 are present in the ICM of WT (arrowhead), but not in clom39. (C) At 36 hpf, expression of Dr.194 96.1.A1 is weakly detected in vasculature and in circulating erythrocytes (arrowheads) of WT embryos, but almost completely absent in clom39. Additional expression of Dr.194 96.1.A1 in epidermal cells, in the brain, and in the eye is present in both WT and clom39. (D) Higher magnification of panel C. Transcripts of Dr.194 96.1.A1 are detected in circulating erythrocytes (arrowhead) on the yolk sac of WT, but not of clom39 embryos at 36 hpf.
Early blood and vascular genes
The detection of developmentally regulated genes was achieved by analyzing gene expression profiles at 2 different embryonic stages. A group of early regulated genes was identified by their stronger down-regulation in clo at 14 somites than at 36 hpf (Figure 2A). Annotation revealed that 11 of these 21 early regulated genes encode transcription factors, the majority of which play most critical roles during early formation of blood and blood vessels. The cluster included scl, lmo2 and hhex, hemangioblast markers that are considered to act immediately downstream of the clo gene,24,41,42 and cmyb, pu.1, and ikaros,4,43,44 which are expressed in hematopoietic progenitor cells. The detection of these well-characterized transcription factors demonstrates the high sensitivity of our approach for capturing genes that play important roles during initial stages of hematopoietic and vascular development.
For some genes in the early regulated cluster, such as Dr.194 96.1.A1, a role in hematopoietic or vascular tissue formation had not been reported. We investigated its expression further by in situ hybridization and found transcripts of Dr.194 96.1.A1 in the ICM of WT, but not in those of clo embryos at the 14-somite stage (Figure 2B), confirming the microarray analysis. Similarly, weak expression for Dr.194 96.1.A1 was detected in erythrocytes and in the vasculature of WT embryos at 36 hpf, yet transcripts were completely absent in clo embryos (Figure 2C-D), suggesting a role for Dr.194 96.1.A1 in both erythroid and vascular development. In summary, genes regulating early stages of hematopoietic and vascular development, such as scl, lmo2, or the novel hematopoietic and vascular progenitor gene Dr.194 96.1.A1, can be specifically identified by whole-embryo microarray analysis.
Erythroid genes
As all examined mutants lack proper red blood cell development, the cluster of genes with globally decreased expression at 36 hpf was linked to the erythroid lineage (Figure 3A). The expression response in clo at 14 somites enabled the separation of early from late erythroid genes. Among those early regulated genes were transcription factors such as gata1, drl, and klf4, whereas klfd, tbx6, and hif1a were grouped in the late cluster, consistent with their temporal expression and function during erythropoiesis.15,17,45-48 Two novel hematopoietic transcription factors, znfl248 and ncoa449 (for further annotation see Table 1), for which expression in erythroid cells had not been previously reported, were also detected and identified as early and late erythroid markers, respectively. In addition to transcription factors, this cluster also contained enzymatic and structural proteins with established functions in erythropoiesis, including jak2a, alas2, band3/slc4a1, and various hemoglobins,13,20,50 as well as genes with novel hematopoietic functions in zebrafish, such as nomo2, mbnl1, abcb6, bzrp, and prdx1, or Dr.231 55.1.A1 and Dr.164 96.1.A1.
Representative genes of various clusters
Gene symbol . | Gene name . |
|---|---|
| Early regulated cluster | |
| scl | T-cell acute lymphocytic leukemia 1 |
| lmo2 | LIM domain only 2 |
| hhex | hematopoietically expressed homeobox |
| fn1 | fibronectin 1 |
| cmyb | transcription factor cmyb |
| spil | Transcription factor PU.1 |
| znfn1a1 | zinc finger protein, subfamily 1A, 1 (Ikaros) |
| Dr. 19496.1.A1 | Dr.19496.1.A1 |
| mmp13 | matrix metalloproteinase 13 |
| sox9b | SRY-box containing gene 9b |
| Erythroid cluster | |
| gata1 | GATA-binding protein 1 |
| drl | draculin |
| klf4 | Kruppel-like factor 4 |
| klfd | Kruppel-like factor d |
| tbx6 | T-box gene 6 |
| hif1a | hypoxia-inducible factor 1a |
| znfl2 | Zinc finger-like gene 2 |
| ncoa4 | nuclear receptor coactivator 4; RET-activating gene ELE1 |
| jak2a | Janus kinase 2a |
| alas2 | aminolevulinate, delta-, synthetase 2 |
| slc4a1 | solute carrier family 4, anion exchanger, member 1 |
| nomo2 | Nodal modulator 2 |
| mbnl1 | Muscleblind-like 1 |
| abcb6 | ATP-binding cassette, sub-family B (MDR/TAP), member 6 |
| bzrp | benzodiazepine receptor, peripheral-type |
| prdx1 | probable thioredoxin peroxidase |
| Dr.23155.1.A1 | Dr.23155.1.A1_at |
| Dr.16496.1.A1 | Dr.16496.1.A1_at |
| nt5c2 | 5′-nucleotidase |
| tfr1a | Transferrin Receptor 1a |
| Myeloid cluster | |
| mpx | myeloid-specific peroxidase |
| ptpn6 | protein tyrosine phosphatase, non-receptor type 6 |
| coro1a | coronin, actin binding protein, 1A |
| npsn | nephrosin |
| lyz | lysozyme |
| lcp1 | lymphocyte cytosolic plastin 1 |
| cnfn | cornifelin |
| laptm5 | lysosomal-associated multitransmembrane protein |
| f13a1 | Factor XIII |
| ncb5or | NADPH cytochrome B5 oxidoreductase |
| slc22a4 | solute carrier family 22, member 4 |
| Dr.2999.1.S1 | chromosome 13 open reading frame 17 |
| pecam1 | platelet/endothelial cell adhesion molecule |
| hspc142 | hypothetical protein HSPC142 |
| dab2 | disabled homolog 2 |
| Vascular cluster | |
| tie1 | endothelium-specific receptor tyrosine kinase 1 |
| flt4 | fms-related tyrosine kinase 4 |
| kdr | kinase insert domain receptor |
| fli1 | Friend leukemia integration 1 |
| dusp5 | dual specificity phosphatase 5 |
| mical-12 | MICAL-like 2 isoform 2 |
| eltd1 | EGF, latrophilin and seven transmembrane domain containing 1 |
| aqp1 | erythrocyte integral membrane protein 28K |
| erg | v-ets erythroblastosis virus E26 oncogene like (avian) |
| mrc1 | Macrophage mannose receptor [Precursor] |
| yrk | Yes-related kinase |
| Upregulated cluster | |
| cyp51 | cytochrome P450, member 51 |
| hmgcs1 | Hydroxymethylglutaryl-CoA synthase, cytoplasmic |
| sc4mol | sterol-C4-methyl oxidase-like |
| fdps | famesyl diphosphate synthase |
| fasn | fatty-acid synthase |
| acat2 | acetyl-CoA acetyltransferase 2 |
| s5des | delta7-sterol-C5-desaturase |
| insig1 | insulin induced gene 1 |
| scarb2 | scavenger receptor class B, member 2 |
Gene symbol . | Gene name . |
|---|---|
| Early regulated cluster | |
| scl | T-cell acute lymphocytic leukemia 1 |
| lmo2 | LIM domain only 2 |
| hhex | hematopoietically expressed homeobox |
| fn1 | fibronectin 1 |
| cmyb | transcription factor cmyb |
| spil | Transcription factor PU.1 |
| znfn1a1 | zinc finger protein, subfamily 1A, 1 (Ikaros) |
| Dr. 19496.1.A1 | Dr.19496.1.A1 |
| mmp13 | matrix metalloproteinase 13 |
| sox9b | SRY-box containing gene 9b |
| Erythroid cluster | |
| gata1 | GATA-binding protein 1 |
| drl | draculin |
| klf4 | Kruppel-like factor 4 |
| klfd | Kruppel-like factor d |
| tbx6 | T-box gene 6 |
| hif1a | hypoxia-inducible factor 1a |
| znfl2 | Zinc finger-like gene 2 |
| ncoa4 | nuclear receptor coactivator 4; RET-activating gene ELE1 |
| jak2a | Janus kinase 2a |
| alas2 | aminolevulinate, delta-, synthetase 2 |
| slc4a1 | solute carrier family 4, anion exchanger, member 1 |
| nomo2 | Nodal modulator 2 |
| mbnl1 | Muscleblind-like 1 |
| abcb6 | ATP-binding cassette, sub-family B (MDR/TAP), member 6 |
| bzrp | benzodiazepine receptor, peripheral-type |
| prdx1 | probable thioredoxin peroxidase |
| Dr.23155.1.A1 | Dr.23155.1.A1_at |
| Dr.16496.1.A1 | Dr.16496.1.A1_at |
| nt5c2 | 5′-nucleotidase |
| tfr1a | Transferrin Receptor 1a |
| Myeloid cluster | |
| mpx | myeloid-specific peroxidase |
| ptpn6 | protein tyrosine phosphatase, non-receptor type 6 |
| coro1a | coronin, actin binding protein, 1A |
| npsn | nephrosin |
| lyz | lysozyme |
| lcp1 | lymphocyte cytosolic plastin 1 |
| cnfn | cornifelin |
| laptm5 | lysosomal-associated multitransmembrane protein |
| f13a1 | Factor XIII |
| ncb5or | NADPH cytochrome B5 oxidoreductase |
| slc22a4 | solute carrier family 22, member 4 |
| Dr.2999.1.S1 | chromosome 13 open reading frame 17 |
| pecam1 | platelet/endothelial cell adhesion molecule |
| hspc142 | hypothetical protein HSPC142 |
| dab2 | disabled homolog 2 |
| Vascular cluster | |
| tie1 | endothelium-specific receptor tyrosine kinase 1 |
| flt4 | fms-related tyrosine kinase 4 |
| kdr | kinase insert domain receptor |
| fli1 | Friend leukemia integration 1 |
| dusp5 | dual specificity phosphatase 5 |
| mical-12 | MICAL-like 2 isoform 2 |
| eltd1 | EGF, latrophilin and seven transmembrane domain containing 1 |
| aqp1 | erythrocyte integral membrane protein 28K |
| erg | v-ets erythroblastosis virus E26 oncogene like (avian) |
| mrc1 | Macrophage mannose receptor [Precursor] |
| yrk | Yes-related kinase |
| Upregulated cluster | |
| cyp51 | cytochrome P450, member 51 |
| hmgcs1 | Hydroxymethylglutaryl-CoA synthase, cytoplasmic |
| sc4mol | sterol-C4-methyl oxidase-like |
| fdps | famesyl diphosphate synthase |
| fasn | fatty-acid synthase |
| acat2 | acetyl-CoA acetyltransferase 2 |
| s5des | delta7-sterol-C5-desaturase |
| insig1 | insulin induced gene 1 |
| scarb2 | scavenger receptor class B, member 2 |
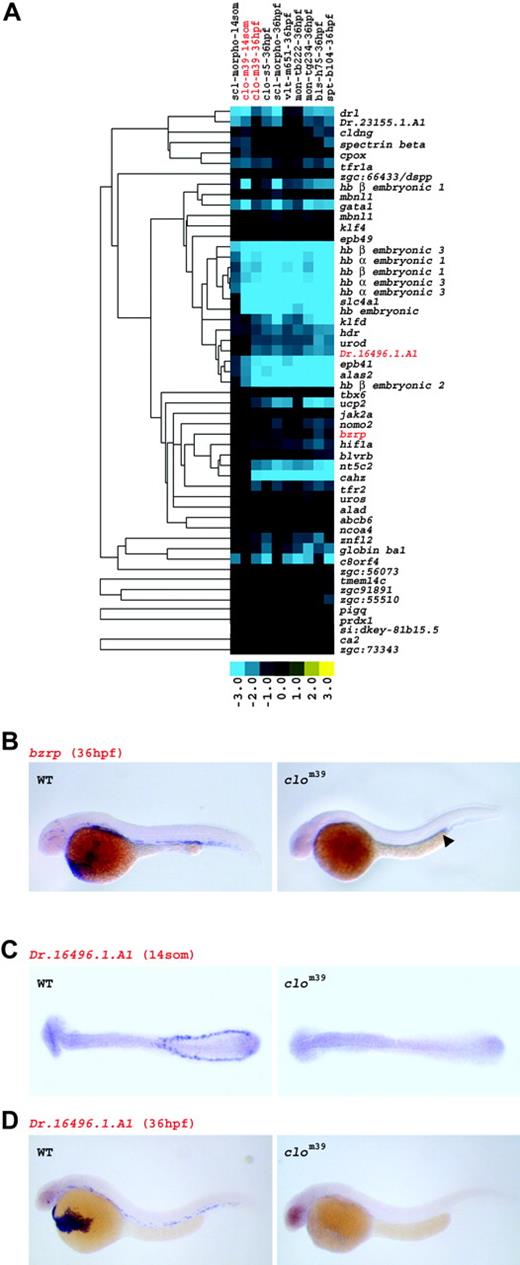
The grouping of genes into the `erythroid cluster' based on their differential expression in WT and mutant embryos was verified by examining their temporal and spatial expression by in situ hybridization. Consistent with the microarray results, bzrp showed strong expression in circulating red blood cells in WT embryos at 36 hpf. Transcripts for bzrp were almost entirely absent in clom39 at 36 hpf, though low levels of expression could be detected posterior to the yolk tube extension, in a region known to express hematopoietic and vascular genes in clo mutants24 (Figure 3B).
Evaluation of the spatiotemporal expression of Dr.164 96.1.A1, a novel erythroid gene, revealed transcripts for Dr.164 96.1.A1 in the ICM at 14 somites and in circulating red blood cells at 36 hpf in WT embryos. In clom39, Dr.164 96.1.A1 expression was completely absent at both stages (Figure 3C-D). Similar results were obtained for the known genes tfr1a and nt5c2, as well as for the novel hematopoietic transcription factors ncoa4 and znfl2 (data not shown and Table S1). Taken together, the down-regulation of expression among all the mutants studied is representative of erythroid genes, such as transcription factors as well as mature red blood cell genes.
Genes down-regulated in all mutants are expressed in erythroid cells. (A) Expression profile of the erythroid gene set. As erythropoiesis is disrupted in all mutants and in sclmo embryos, the set of globally down-regulated genes at 36 hpf was linked to the erythroid lineage. Columns represent mutants of which name, tested allele, and the respective developmental stage are given; rows represent genes. Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos. The expression of genes highlighted in red is illustrated by in situ hybridization in panels B, C, and D. (B) Expression of bzrp in WT and in clom39 embryos at 36 hpf. Transcripts of bzrp are abundantly detected in circulating erythrocytes in WT, but only in a limited number of cells in the trunk around the posterior part of the yolk tube extension in clom39 (arrowhead). (C) Transcripts of Dr.164 96.1.A1 are present in cells of the ICM of WT embryos, but absent in clom39 (both shown at 14-somite stage). (D) At 36 hpf, Dr.164 96.1.A1 is expressed in circulating erythrocytes in WT, but not in clom39 embryos.
Genes down-regulated in all mutants are expressed in erythroid cells. (A) Expression profile of the erythroid gene set. As erythropoiesis is disrupted in all mutants and in sclmo embryos, the set of globally down-regulated genes at 36 hpf was linked to the erythroid lineage. Columns represent mutants of which name, tested allele, and the respective developmental stage are given; rows represent genes. Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos. The expression of genes highlighted in red is illustrated by in situ hybridization in panels B, C, and D. (B) Expression of bzrp in WT and in clom39 embryos at 36 hpf. Transcripts of bzrp are abundantly detected in circulating erythrocytes in WT, but only in a limited number of cells in the trunk around the posterior part of the yolk tube extension in clom39 (arrowhead). (C) Transcripts of Dr.164 96.1.A1 are present in cells of the ICM of WT embryos, but absent in clom39 (both shown at 14-somite stage). (D) At 36 hpf, Dr.164 96.1.A1 is expressed in circulating erythrocytes in WT, but not in clom39 embryos.
Myeloid genes
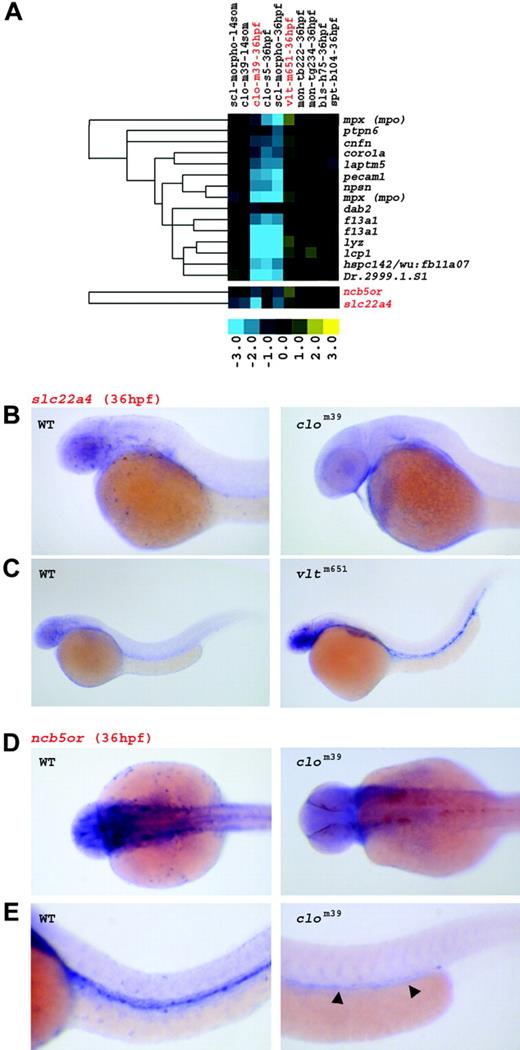
Two clusters contained genes that were down-regulated in clo, but up-regulated in vlt embryos, and upon annotation, identified myeloid or monocytic markers, such as mpx, ptpn6, coro1a, npsn, lyz, and lcp14,46,51 (Figure 4A). These clusters also contained cnfn, laptm5, f13a1, ncb5or, slc22a4, and Dr.2999.1.S1, genes for which a hematopoietic function had not been demonstrated. When evaluated by in situ hybridization in WT or vlt mutants (Figure 4B-C), slc22a4 showed a highly restricted expression pattern that is typical of myeloid cells. Transcripts of this gene were not detected in clom39 embryos at 36 hpf. ncb5or showed similar myeloid expression, but was additionally found in axial and intracranial vessels in WT embryos (Figure 4D-E). Consistent with the vascular defect of clo mutants, ncb5or was observed only in vascular cells of the trunk near the posterior part of the yolk tube extension in clom39 embryos.
Three genes in this cluster, pecam1, hspc142, and dab2, although down-regulated in clo and sclmo embryos at 36 hpf, were not significantly regulated in vlt mutants. Instead, this pattern indicated specific endothelial expression, which was confirmed when 2 of these probes were examined by in situ hybridization (pecam1 [data not shown] and dab251 ). These findings further demonstrated that the up-regulation of gene expression in vlt is inherent to myeloid cells.
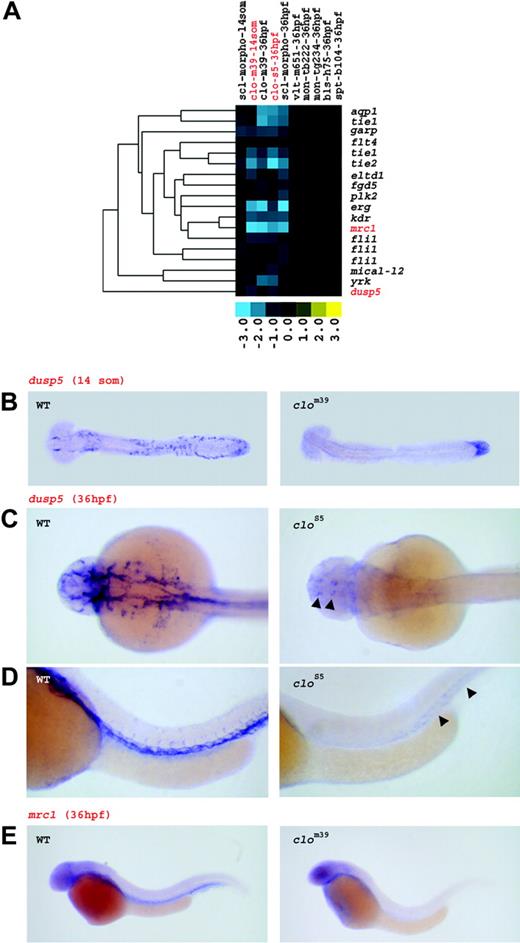
Vascular genes
clo and sclmo embryos are characterized by impaired vasculogenesis and angiogenesis, respectively. Consistent with these defects, the putative vascular cluster comprised genes that were down-regulated in clo mutants and in sclmo embryos, but were not affected in the other mutants (Figure 5A). Annotation demonstrated that 10 of the 18 genes in this cluster represented known zebrafish endothelial cell markers, such as tie1, flt4, kdr, fli1, dusp5, or mical-l2.8,45,46,52-54 However, several novel vascular zebrafish genes, for instance eltd1, aqp1, erg, mrc1, and yrk, were additionally identified.
At the 14-somite stage, angioblasts are absent in the ICM of clo, but present in sclmo embryos.25 Consistent with this, we observed significant differential expression of endothelial genes, such as tie1, flk1, mrc1, yrk, and dusp5, between clo and WT embryos, but not between sclmo and uninjected embryos at the 14-somite stage.
Genes up-regulated in vlt at 36 hpf represent myeloid genes. (A) Genes down-regulated in clo and sclmo embryos, up-regulated in vlt, but unaffected in the other mutants, were linked to the myeloid lineage. Columns represent mutants of which name, tested allele, and the respective developmental stage are given; rows represent genes. Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos. The expression of genes highlighted in red is further illustrated by in situ hybridization in panels B, C, D, and E. (B,C) Expression of slc22a4 in WT, clom39, and vltm651 embryos at 36 hpf; transcripts are highly restricted to myeloid cells in WT, abundant in circulating myeloid cells of vltm651 embryos, but absent in clom39 mutants. (D,E) In WT embryos, ncb5or is expressed in myeloid cells and in vasculature at 36 hpf. clom39 mutants show only low levels of transcription in angioblasts around the posterior region of the yolk tube extension (arrowheads). Panel D shows dorsal view; panel E shows lateral view.
Genes up-regulated in vlt at 36 hpf represent myeloid genes. (A) Genes down-regulated in clo and sclmo embryos, up-regulated in vlt, but unaffected in the other mutants, were linked to the myeloid lineage. Columns represent mutants of which name, tested allele, and the respective developmental stage are given; rows represent genes. Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos. The expression of genes highlighted in red is further illustrated by in situ hybridization in panels B, C, D, and E. (B,C) Expression of slc22a4 in WT, clom39, and vltm651 embryos at 36 hpf; transcripts are highly restricted to myeloid cells in WT, abundant in circulating myeloid cells of vltm651 embryos, but absent in clom39 mutants. (D,E) In WT embryos, ncb5or is expressed in myeloid cells and in vasculature at 36 hpf. clom39 mutants show only low levels of transcription in angioblasts around the posterior region of the yolk tube extension (arrowheads). Panel D shows dorsal view; panel E shows lateral view.
To confirm that the differential gene regulation in this cluster was due to vascular expression, 2 genes were further examined by in situ hybridization. In WT embryos, transcripts for dusp5 were detected in the ICM at 14 somites and in the entire arterial and venous system at 36 hpf, whereas clo embryos displayed markedly reduced expression of dusp5, which was limited to the tailbud region at 14 somites and to distinct areas in the brain and in the trunk at 36 hpf (Figure 5B-D). When additionally evaluated in sclmo at 36 hpf, dusp5 was present in the vasculature, yet at reduced expression levels in comparison to WT embryos (Figure S1). This finding is in agreement with the previously described angiogenesis defect in sclmo at later stages of embryonic development.25 The novel vascular marker mrc1 showed similar expression in blood vessels of WT embryos at 36 hpf, and was absent in clo at 36 hpf (Figure 5E). In sum, the gene expression of this cluster demonstrates that novel and known vascular genes are specifically down-regulated in clo embryos at 14 somites and in clo and sclmo embryos at 36 hpf.
Genes down-regulated in clo and sclmo embryos show vascular expression pattern. (A) Expression profile of the vascular gene set. The set of genes specifically down-regulated in clo at 14 somites and in clo and sclmo at 36 hpf was linked to the vasculature. Columns represent mutants of which name, tested allele, and the respective developmental stage are given; rows represent genes. Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos. The expression of genes highlighted in red is further illustrated by in situ hybridization in panels B, C, D, and E. (B) Expression of dusp5 in WT and in clom39 embryos at 14 somites. Transcripts are found in ICM cells in WT, but are nearly absent in clom39 mutants; low levels of expression are only present in the tail bud. (C,D) Expression of dusp5 at 36 hpf. dusp5 transcripts are detected throughout the entire arterial and venous system in WT embryos. In clos5 embryos, expression is limited to a small area in the brain and to the posterior trunk (arrowheads). Panel C shows dorsal view; panel D shows lateral view. (E) mrc1 is expressed in the vasculature of WT embryos at 36 hpf, but is almost entirely absent in clom39.
Genes down-regulated in clo and sclmo embryos show vascular expression pattern. (A) Expression profile of the vascular gene set. The set of genes specifically down-regulated in clo at 14 somites and in clo and sclmo at 36 hpf was linked to the vasculature. Columns represent mutants of which name, tested allele, and the respective developmental stage are given; rows represent genes. Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos. The expression of genes highlighted in red is further illustrated by in situ hybridization in panels B, C, D, and E. (B) Expression of dusp5 in WT and in clom39 embryos at 14 somites. Transcripts are found in ICM cells in WT, but are nearly absent in clom39 mutants; low levels of expression are only present in the tail bud. (C,D) Expression of dusp5 at 36 hpf. dusp5 transcripts are detected throughout the entire arterial and venous system in WT embryos. In clos5 embryos, expression is limited to a small area in the brain and to the posterior trunk (arrowheads). Panel C shows dorsal view; panel D shows lateral view. (E) mrc1 is expressed in the vasculature of WT embryos at 36 hpf, but is almost entirely absent in clom39.
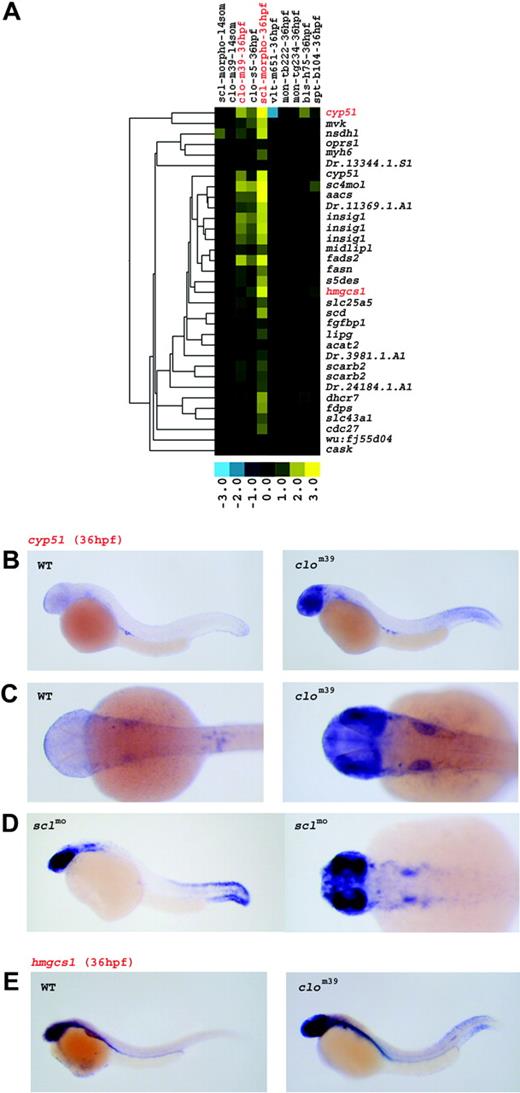
Genes up-regulated in clo and in sclmo embryos
An additional cluster grouped 33 genes that showed similar up-regulation in clo and sclmo embryos at 36 hpf (Figure 6A). Strikingly, the majority of these genes have previously been implicated in fatty acid and isoprenoid synthesis. These included cyp51, hmgcs1, sc4mol, fdps, fasn, acat2, s5des, and insig,55 as well as scarb2, for which a role in cellular cholesterol release has been reported.56
Three representative genes, cyp51, hmgcs1, and sc4mol, were examined by in situ hybridization. Transcripts for cyp51 in 36 hpf WT embryos were found in germ cells57 and at low levels in the eye and in epidermal cells of the forehead and the caudal fin. In contrast, expression of cyp51 in clom39 and in sclmo, though equally expressed in germ cells, was strongly up-regulated in the eye, in epidermal cells of the forehead and of the caudal fin, and in the brain (Figure 6B-D). A similar spatial expression pattern was found for hmgcs1 and sc4mol (Figure 6E and Figure S2, respectively). WT embryos at 36 hpf expressed only low levels of hmgcs1 or sc4mol, primarily in the eye and in epidermal cells, whereas in clom39 at 36 hpf, transcripts were abundantly detected in the eye, in the brain, and in epidermal cells of the head and of the tail fin. These findings describe unique gene regulations at 36 hpf and define thereby a novel phenotype of clo and sclmo embryos.
Comparison with in situ expression
In situ hybridization of selected novel genes strongly supported that the clusters with decreased expression specifically demarcated hematopoietic and vascular genes. We further probed the specificity of our approach by comparing our microarray analysis results to in situ hybridization expression data obtainable through PubMed, The Zebrafish Information Network,46 and unpublished results (G.J.W. and R.A. Wingert, January 2005) available in our laboratory (Table S1). This comparison revealed that among 247 genes found to be consistently down-regulated in clo during at least 1 developmental stage, 136 have been tested by in situ hybridization. Of these 136 genes, 83 have reported hematopoietic or vascular expression. A very high percentage (87%; 72/83) of these genes localized to the 4 selected clusters and matched the lineage-specific expression determined by our microarray analysis. These findings provided further evidence that whole-embryo microarray analysis detected hematopoietic and vascular gene regulation with high specificity.
Discussion
The zebrafish is an excellent developmental and genetic system for investigating hematopoiesis. The availability of mutants with defects at distinct stages of blood formation is of particular advantage. Although there are many methods to characterize and compare these mutants, our work here establishes microarrays as a rapid and informative method to assess gene regulation. Our microarray analysis has further characterized the bloodless mutant phenotypes by identifying novel hematopoietic genes and by assigning specific genes to different cell lineages during hematopoiesis and vasculogenesis. This comparative approach of examining mutant zebrafish strains and evolutionarily conserved gene modules is an extremely useful tool for assembling the hematopoietic and vascular transcriptional program.
Isoprenoid synthesis genes are up-regulated in the head and tail of clo and sclmo embryos. (A) Expression profile of the genes up-regulated in clo and sclmo embryos at 36 hpf. Columns represent mutants of which name, tested allele, and the respective developmental stage are given; rows represent genes. Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos. The expression of genes highlighted in red is further illustrated by in situ hybridization in panels B, C, D and E. (B-D) Expression of cyp51 at 36 hpf. In WT, transcripts are found in germ cells and at low levels in epidermal cells of the forehead and of the tail fin; in clom39 embryos, abundant expression of cyp51 is detected primarily in the eye, in the brain, and in epidermal cells of the forehead and the tail fin. Panel B shows lateral view; panel C shows dorsal view. (D) sclmo embryos at 36 hpf show an expression pattern similar to clo, with transcripts of cyp51 abundant in the eye, in epidermal cells of the forehead and the tail fin, and around the otic placode. (E) WT embryos express only low levels of hmgcs1 in the brain and the eye at 36 hpf. In clom39 mutants at 36 hpf, hmgcs1 is highly expressed in the brain, in the eye, and in epidermal cells of the caudal fin.
Isoprenoid synthesis genes are up-regulated in the head and tail of clo and sclmo embryos. (A) Expression profile of the genes up-regulated in clo and sclmo embryos at 36 hpf. Columns represent mutants of which name, tested allele, and the respective developmental stage are given; rows represent genes. Fold changes are shown as log2 (mutant/WT, or morphant/uninjected). Blue and yellow boxes indicate decreased and increased gene expression, respectively, in mutant compared with WT (morphant compared with uninjected) embryos. The expression of genes highlighted in red is further illustrated by in situ hybridization in panels B, C, D and E. (B-D) Expression of cyp51 at 36 hpf. In WT, transcripts are found in germ cells and at low levels in epidermal cells of the forehead and of the tail fin; in clom39 embryos, abundant expression of cyp51 is detected primarily in the eye, in the brain, and in epidermal cells of the forehead and the tail fin. Panel B shows lateral view; panel C shows dorsal view. (D) sclmo embryos at 36 hpf show an expression pattern similar to clo, with transcripts of cyp51 abundant in the eye, in epidermal cells of the forehead and the tail fin, and around the otic placode. (E) WT embryos express only low levels of hmgcs1 in the brain and the eye at 36 hpf. In clom39 mutants at 36 hpf, hmgcs1 is highly expressed in the brain, in the eye, and in epidermal cells of the caudal fin.
Whole-embryo microarray analysis captured differential expression not only of the earliest transcription factors, such as scl, lmo2, and gata1, but also of genes expressed in differentiated blood cells. Moreover, our analysis led to the identification of 31 genes with novel hematopoietic or vascular expression patterns in zebrafish, including the early blood and vascular marker Dr.194 96.1.A1, or the erythroid transcription factors znfl2 and ncoa4. When selected novel genes were further examined by in situ hybridization, their vascular- or blood lineage–specific expression was confirmed in every case (13/13, Table S1).
Our in situ hybridization results, in combination with other studies, confirmed for 61 of 65 genes (94%, Table S1) the erythroid-, myeloid-, or vascular-specific expression predicted by the microarray data. Together, these findings highlight the remarkable sensitivity and specificity of whole-embryo microarray analysis.
The extremely low number of false positives in our study may be due to a few factors. First, the comparison of WT embryos to mutants lacking blood cell lineages resulted in extremely large fold changes for blood genes. As the test statistic contains the fold change in the numerator, blood genes are therefore likely to be statistically detected. Second, the candidate gene sets were selected using multiple criteria: (1) significant differential expression in clo mutants, (2) passing the filters for inclusion in the clustergram, and (3) a specific expression pattern in multiple genotypes in the clustergram. Taken together, these criteria are extremely stringent and could reasonably result in a very low false-positive rate.
Our results demonstrate that whole-embryo microarray analysis also has the sensitivity required to detect blood cell–specific changes of genes that are expressed in several tissues. For example, the transcription factor klf4 was down-regulated in the erythroid cluster in clo, despite having WT expression in the hatching gland and in the lateral line ganglia of this mutant.45 Similarly, cmyb is abundantly transcribed in the eye and in mucous cells of clo,8 and fn1 is expressed throughout the mesoderm and in the tailbud of clo at 14 somites, but was identified as down-regulated in the early hematopoietic and vascular cluster only by its reduced endocardial expression.58 These results demonstrate that the comparison of whole WT and mutant zebrafish embryos is a powerful method for the global detection of hematopoietic genes that might also be expressed in nonhematopoietic tissues.
Improved analysis of mutant phenotypes
Our evaluation of mutants by gene expression profiling enables extensive analysis of mutant phenotypes and has yielded several novel findings. For instance, vlt, mon, and bls appear to have very similar erythropoietic defects. Gene expression profiling, however, reveals a clear up-regulation of known and also novel myeloid genes in vlt with respect to mon and bls. This finding is consistent with recent studies in which cells in the ICM of vlt mutants were found to shift toward the myeloid lineage and express mpo and l-plastin in the absence of gata1.43,59
An additional novel trait was identified in the clo mutant and sclmo, both of which have extensively described hematopoietic and vascular defects. The novel gene expression phenotype is characterized by the strong up-regulation of isoprenoid and fatty acid synthesis genes in the brain, in the eye, and in epidermal cells. It is possible that this expression pattern represents a response to the angiogenesis defect in clo mutants and sclmo. Fatty acid synthase has recently been shown to confer a selective growth advantage to cancer cells in response to stresses such as hypoxia and/or low pH.60 Alternatively, the up-regulation of isoprenoid genes might represent a response of migrating and growing cells to loss of clo and/or scl: HMG-CoA receptor activity is required for proper germ cell migration,57 and a role for genes involved in isoprenoid biosynthesis has also been suggested in axon growth.61,62 Interestingly, other genes known to be expressed by migrating cells, such as fn1,58 mmp13, mmp9,63 the ortholog of Dr.155 75.1.A1(uPAR),58 or sox9b,64 are also significantly regulated in clo (Table S1). The increased expression of isoprenoid synthesis genes in clo mutants might therefore provide crucial insight into the mechanism by which clo regulates hematopoiesis and vasculogenesis.
Novel hematopoietic and vascular genes
The novel gene Dr.194 96.1.A1 was detected in progenitor cells of the ICM, in circulating erythrocytes, and in vascular tissue. Sequence analysis shows that this gene contains a meprinlike domain, suggesting a possible function for Dr.194 96.1.A1 during vessel formation. Genes with novel erythroid expression included the 2 transcription factors ncoa4 and znfl2, as well as nomo2 and abcb6. ncoa4 reportedly activates nuclear factor (NF)–kappaB and induces proinflammatory pathways in thyroid epithelial cells,65 and znfl2 acts during formation of the posterior central nervous system in zebrafish48 ; erythropoietic expression for these 2 transcription factors, however, had not been determined. In zebrafish, nomo2 is expressed during mesendodermal patterning and antagonizes Nodal- and Activin-dependent signaling,66 which may provide insight into its hematopoietic function. For abcb6, a role in mitochondrial regulation of iron homeostasis and Fe/S cluster assembly is known,67 but a function during erythropoiesis has not been described.
Among the myeloid genes was slc22a4, which encodes an organic solute transporter. slc22a4 is regulated by the hematopoietic transcription factor runx168 and has been implicated in the pathogenesis of autoimmunologic disorders such as rheumatoid arthritis and Crohn disease.68,69 The coexpression of the novel myeloid gene ncb5or in myeloid and vascular cells suggests a similar function in both cell types. The vascular cluster included the endothelial gene mrc1, which encodes a cell surface receptor in monocytic cells that recognizes glycoproteins.
The isolation of novel genes and previously characterized genes with unknown roles in hematopoiesis or vasculogenesis highlights the impact of gene expression profiling in zebrafish on furthering our understanding of the hematopoietic process. Additional characterization of these genes in zebrafish using morpholinos, overexpression, or directed mutagenesis will establish functions for these genes during blood and vascular development.
Further applications
Comparison of WT and mutant embryos at 14 somites and at 36 hpf revealed both early- and late-specific markers. Extending this microarray analysis to a larger number of developmental stages will help to establish the stage-specific gene expression of stem cells, progenitors, precursors, and the terminal blood lineages.
Transcriptional analyses of specific lineages in the zebrafish are not limited to whole-embryo experiments and can be performed, for instance, on 2 blood cell populations from transgenic zebrafish (eg, lmo2+gfp,28 and gata1+gfp cells). Such comparisons, however, lack the advantage of whole-embryo microarray analysis to detect compensatory gene regulations in nonhematopoietic tissues. As these effects occur in response to the genetic defect, they facilitate a comprehensive view of the mutant phenotype.
Future studies examining the transcriptional profile of additional mutants and morphants promise to identify other mutant-specific expression patterns. For instance, preliminary analysis of mon mutants reveals a gene set that is expressed consistently with its mesenchymal defect. Other mutants with uncharacterized hematopoietic regulation can also be rapidly screened by comparing their gene expression profile to our data. Analogous comparisons of differential gene regulation in mouse knockouts should provide a similarly rich resource for understanding hematopoiesis.
In summary, gene expression profiling of zebrafish blood mutants is a highly efficient method for evaluating tissue development at the transcriptional level. By comparing the individual mutant profiles, we can identify stage-specific patterns, discover unknown hematopoietic and vascular genes, and establish pathways that are specific to mutant phenotypes. This approach represents a powerful tool for defining the genetic program of vertebrate hematopoiesis and vaculogenesis.
Prepublished online as Blood First Edition Paper, April 12, 2005; DOI 10.1182/blood-2004-11-4541.
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases/Stem Cell Genome Anatomy Project (1U01 DK63 328) and from the German Research Foundation (DFG WE 2907/1-1). L.I.Z. is an investigator of the Howard Hughes Medical Institute.
G.J.W. designed and performed research, analyzed data, and wrote the paper; S.E.C. contributed analytical tools and analyzed data; K.A.D. and N.N.P.L. performed research; Y.Z. and L.I.Z. designed research.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Alan J. Davidson, Rebecca A. Wingert, and Nelson Hsia for critical comments and thoughtful suggestions during the preparation of the manuscript. We also thank Chad Dow, Ken Chiang, Nicole Al-Greene, Trista North, Kamden Kopani, and Clayton Kinney for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal