Abstract
Nucleosome assembly proteins (NAPs) bind core histones, facilitate chromatin remodeling, and can act as transcriptional coactivators. We previously described the isolation of a Xenopus NAP1-like (xNAP1L) cDNA, which encodes a member of this protein family. Its zygotic expression is restricted to neural cells, the outer cells of the ventral blood island (VBIs), and the ectoderm overlying the blood precursors. Here, we report that depletion of zygotic xNAP1L in embryos produces no obvious morphologic phenotype, but ablates α-globin mRNA expression in the VBIs. Transcript levels of the hematopoietic precursor genes SCL and Xaml (Runx-1) are also reduced in the VBIs. SCL expression can be rescued by injection of xNAP1L mRNA into the ectoderm, showing that the effect of xNAP1L can be non–cell autonomous. Fli1 and Hex, genes expressed in hemangioblasts but subsequently endothelial markers, were unaffected, suggesting that xNAP1L is required for the hematopoietic lineage specifically. Our data are consistent with a requirement for xNAP1L upstream of SCL, and injection of SCL mRNA into xNAP1L-depleted embryos rescues α-globin expression. Thus, xNAP1L, which belongs to a family of proteins previously believed to have general roles, has a specific function in hematopoiesis.
Introduction
Nucleosome assembly protein (NAP) family members are found in all eukaryotes,1 and, although primary sequence varies considerably among them, they share the ability to assemble nucleosomes in vitro.2-7 The NAPs have been assigned various functions; for example, yeast NAP1-like (NAP1L) has been shown to interact with both histones H2A and H2B and their karyopherins, facilitating their transport across the nuclear membrane.8 In support of a similar histone shuttling role for NAPs in other species, both Drosophila NAP1 and human NAP1L4 are nuclear during S phase and cytoplasmic during G2.5,7 There is also evidence that NAPs have a role in control of the cell cycle, both yeast NAP1 and a Xenopus NAP interact specifically with B-type cyclins and are phosphorylated by cyclin B/p34cdc2 in vitro.9 Deletion of the NAP1 gene in yeast showed that it is required for the proper function of Clb29,10 and regulates cell cycle in combination with the Gin4 kinase,11 a process that requires nucleo-cytoplasmic shuttling.12
Biochemical evidence suggests a role for NAPs in chromatin remodeling during transcription activation.13-15 This may have significance in vivo since the transcript levels from 10% of yeast genes are altered when yeast NAP is mutated.16 Data also suggest that mammalian NAP proteins might have a more direct role in transcription control via their interactions with the coactivators CBP/p300 and augmentation of p300-dependent transcription, possibly by stabilizing the interactions between it and core histones.14,15,17 hNAP1L, the human ortholog of Xenopus NAP1L (xNAP1L), also interacts with the activation domain of a transcriptional activator, the E2 protein encoded by papillomaviruses. Coexpression with hNAP1L strongly enhances transcriptional activation by E2 and is independent of p300 binding. In the same study, hNAP1L was also shown to interact with the transcription factor p53.18 Vertebrate NAP proteins therefore appear to have an important role in transcriptional activation.
All of the potential roles of NAPs outlined above could influence development, and 2 studies have addressed whether this is the case. Inactivation of NAP1L2 in mice and NAP1 in Drosophila was embryonic lethal19 ; surviving chimeric mouse embryos had surface ectoderm defects in addition to open neural tubes and exposed brains.20 More recently, a human disease has been demonstrated to result from a mutation that leads to a truncation of the testis-specific Y-like (TSDYL) protein immediately upstream of its NAP domain. Sudden infant death with dysgenesis of the tissues syndrome (SIDDT) is characterized by defective development of the male genitalia and as-yet-undefined neural defects leading to death due to sudden cardiorespiratory arrest before 12 months of age.21 Current evidence therefore suggests that NAP proteins are important for development and have multiple, chromatin-related functions, although the precise details of most of these remain to be defined.
In order to discover more about the role of NAPs in development, we isolated the cDNA encoding Xenopus NAP1L, which has 92% amino acid identity to mammalian NAP1L proteins and is grouped closely with this specific subset of NAP proteins by phylogenetic analysis.1 Expression in adults is predominantly in ovaries, and this maternal protein remains a major component of xNAP1L within the embryo until swimming tadpole stages. xNAP1L mRNA is initially expressed throughout the embryo, but by gastrula stages it is found predominantly in the presumptive ectoderm. Later, the mRNA is detected in the neural crest, neural tube, eyes, tailbud, and ventral blood islands (VBIs).1
In Xenopus embryos, as in other vertebrates, hematopoiesis occurs in 2 distinct waves. The first site of blood formation is the VBI, equivalent to the yolk sac in mammals; this is followed by definitive hematopoiesis in which cells originating from the dorsal lateral plate (DLP) mesoderm give rise to all the cell lineages that constitute adult blood.22-24 VBI formation has been well characterized in Xenopus embryos; fate-mapping has shown that the anterior and posterior parts of the VBI are derived from different regions of the blastula embryo.24-26 The blood progenitors then migrate to the most ventral part of the mesoderm where they initially have characteristics of hemangioblasts, cells that give rise to both hematopoietic and endothelial lineages.27
The formation of these hematopoietic precursors depends on a number of signals and transcription factors. Bone morphogenetic protein (BMP) signaling from the ectoderm is required for globin expression in the VBI,27,28 while fibroblast growth factor (FGF) limits the size of the blood islands.29 Transcription factors required for blood development are often useful markers of hematopoietic precursors. The zinc finger protein GATA-2 is expressed ventrally in all 3 germ layers of neurula stage embryos, but later its expression becomes more restricted to the VBI.30,31 Gene disruption experiments indicate that GATA-2 is essential for formation of all hematopoietic cells.32 The basic helix-loop-helix protein stem cell leukemia (SCL) is a transcription factor that can induce blood formation if expressed ectopically in embryos33,34 and is necessary for both hematopoietic stem cell genesis and for erythroid and megakaryocytic differentiation in mice.35 Xaml, the Xenopus homolog of the hematopoietic progenitor transcription factor acute myeloid leukemia (AML)/Runx-1, is also critical for early events in Xenopus blood formation since a dominant interfering mutant of this protein inhibits primitive hematopoiesis.25 Here, we test the effect of depleting zygotic xNAP1L from embryos and show a specific requirement for xNAP1L-dependent signaling in primitive hematopoiesis.
Materials and methods
Morpholino oligonucleotide (MO) injections
Morpholino antisense oligonucleotides were purchased from Gene Tools (Philomath, OR). The sequence of the xNAP1L MO was 5′-TATGTTAGCCATTATGTTGAGCACC-3′ and was designed to overlap the start of translation. The control MO was the inverse of the above sequence: 5′-ATACAATCGGTAATACAACTCGTGG-3′. MO (12 ng) was injected into fertilized Xenopus laevis eggs that were prepared and subsequently cultured as previously described36 ; they were staged according to Nieuwkoop and Faber.37
In vitro translation and immunoprecipitation
Synthetic xNAP1L mRNA was prepared by transcription from a Bluescript plasmid containing xNAP1L cDNA using a mMessage machine kit (Ambion, Austin, TX) and was used to program rabbit reticulocyte (Promega, Madison, WI) in the presence of xNAP1L MO or control MO according to the manufacturer's instructions. 35S-methionine–labeled proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and visualized using a phosphorimager (Fuji). To detect the effect of the MO on zygotic xNAP1L levels, sets of 100 embryos were injected with either control or xNAP1L morpholino at the 1-cell stage and with both synthetic native xNAP1L mRNA and 35S-methionine (> 1000 Ci [37 000 GBq]/mmol; 0.1 μCi [0.0037 MBq] in 4 nL) at the 2-cell stage. After immunoprecipitation with xNAP1L polyclonal antibody,1 the radiolabeled zygotic protein was detected using a phosphorimager (Fuji FLA-5000; Raytech, Sheffield, United Kingdom).
In situ hybridization
Whole-mount in situ hybridization was performed as described previously.38 The probes used were as described in the following publications: αT4-globin30 ; Xaml25 ; XHex39 ; Xfli1,40 and SCL.34 Images were obtained using a Leica Wild M32 steromicroscope (Wetzlar, Germany) with a Nikon DN 100 camera (Kanagrewa, Japan) and handled using Adobe Photoshop (San Jose, CA).
RNA injection
Synthetic xNAP1L mRNA for rescues was prepared by linearization of xNAP1L in the plasmid pButSfi1 with SfiI and subsequent transcription using a mMessage machine kit (Ambion). This mRNA includes only 8 nucleotides of the sequence bound by the MO; the others are replaced by the globin 5′–untranslated region (UTR) and its translation in vitro is unaffected by MO (data not shown). The morphant phenotype was rescued by injecting NAP-depleted embryos with 25 pg of this synthetic xNAP1L mRNA into either the upper tier (ectoderm) or marginal zone (mesoderm) of an 8-cell embryo. Native, synthetic xNAP1L mRNA was prepared by in vitro transcription of our original xNAP1L cDNA in pBluescript1 ; this produces an mRNA identical to the native one, the translation of which is inhibited by the MO. SCL mRNA was made by transcription as described previously.34
RNA isolation and real-time RT-PCR
Eggs from a single female were injected with either control MO or xNAP1L MO and allowed to develop to stage 18 or stage 26. RNA was extracted from embryos or dissected ventral regions using trizol (Gibco, Grand Island, NY) and reverse transcribed with superscript II reverse transcriptase (Gibco). Levels of the mRNAs shown were assayed using the comparative cycle threshold (CT) method by real-time reverse-transcriptase–polymerase chain reaction (RT-PCR) on an ABI 7900HT sequence analyzer (Applied Biosystems, Foster City, CA) using Taqman probes and ornithine decarboxylase (ODC) as a control mRNA for normalization. The probe sequences are as follows: GATA-2 TaqMan, ACCCCGACTCCCATCCACCCTTC; GATA-2 forward, CATTCAGGGCACATCTTGCA; GATA-2 reverse, TTTATCCCATAGCCGTCACCAT; ODC TaqMan, AAGCGCTCCCCCGTGTCACTC; ODC forward, AAAAAGCATGTGCGTTGGTTT; ODC reverse, TCGTTGCATTTTACGGCATAAA; Hex TaqMan, CCTCTGTACCCCTTCTCCAGGCCAGT; Hex forward, CTATGGAGCCAGCACCTATGC; Hex reverse, ATGAGGGCGTGGGTGTAGTC; αT4-globin TaqMan, CCAAGGTTCGTGCCCATGGCAA; aT4-globin forward, TGGCTGCCATCTCTGGAAAT; αT4-globin reverse, TCATCGACGGCAGACAAAAC; AML TaqMan, CAAATCCCATGCCCAACCCACG; AML forward, GGGTCAGCCCCCATCAC; AML reverse, AAAAGCTGCGGAATGATTTAGG; SCL TaqMan, CAGAGCTAAGCGAAGACCTGGCCCC; SCL forward, ACTTTGGTGACCCAGACACCTT; SCL reverse, GGACCTTCAGAGATTTCCACTTCTA; Fli-1 TaqMan, ATCCAAACCCAAATGTGCCACGTCAC; Fli-1 reverse, GTGGGACTGCACATGGGTATTAG; and Fli-1 forward, GGAATTCACCAAGTGCAAACATT.
Results
NAP MO blocks translation of xNAP1L in vitro and in vivo
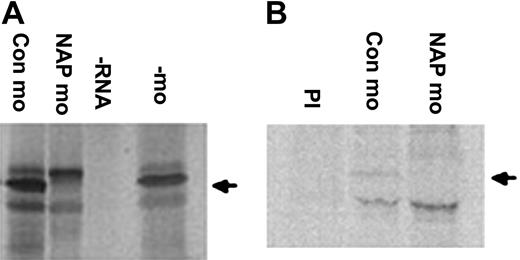
Since one of the sites of xNAP1L mRNA expression is the VBI, the site of primitive hematopoiesis in Xenopus, we tested the effect of perturbing xNAP1L expression on blood formation. Overexpression of xNAP1L produced an increase in α-globin expression.41 Since the investigation of gene function is greatly enhanced by combining both gain and loss of function experiments, we investigated the role of zygotic xNAP1L further by depleting Xenopus laevis embryos of this protein using morpholino oligonucleotides (MOs).42 The MO inhibited translation of xNAP1L mRNA in vitro, while the control MO did not affect xNAP1L levels (Figure 1A). Endogenous zygotic xNAP1L protein is difficult to detect against the high background of maternal protein in embryos, we therefore tested whether the MO was functional in vivo by expression of synthetic, native xNAP1L mRNA in embryos. We injected NAP MO or control MO into fertilized eggs and then overexpressed the zygotic protein by injection of native xNAP1L mRNA at the 2-cell stage and labeled it (to distinguish it from the maternal protein) by coinjection of 35S-methionine. Immunoprecipitation with xNAP1L-specific antibody from control MO–injected embryos produces a labeled protein that migrates at the position of xNAP1L on SDS-PAGE and that is absent in immunoprecipitations with preimmune serum. Injection of the xNAP1L MO into embryos decreased the level of zygotic xNAP1L to the point where it was undetectable (Figure 1B). The MO therefore blocks translation of xNAP1L in vivo. We performed an experiment testing the effect of the MO on the endogenous protein using a similar strategy. Despite using the highest levels of 35S-methionine that the embryos could tolerate, the endogenous xNAP1L was barely detectable; however, it did become undetectable in MO-injected embryos (data not shown).
xNAP1L morpholino oligonucleotide blocks translation of xNAP1L RNA in vitro and in vivo. (A) Rabbit reticulocyte lysate was programmed with xNAP1L mRNA in the absence of MO (–mo) and in the presence of xNAP1L MO (NAP mo) and control MO (Con mo, the inverted NAP sequence); 35S-methionine–labeled proteins were separated by SDS-PAGE and visualized by autoradiography. No translation occurred in the absence of added RNA (–RNA). (B) To detect the effect of xNAP1L MO on xNAP1L protein levels in vivo, embryos were injected with 35S-methionine and xNAP1L mRNA (100 pg) to increase endogenous levels of the protein to detectable levels and either xNAP1L MO or control MO (12 ng). After immunoprecipitation with an xNAP1L polyclonal antibody, the radiolabeled zygotic protein was detected using a phosphorimager. To confirm the specificity of the antibody, an immunoprecipitation with preimmune serum (PI) was performed. The position of xNAP1L protein is shown by an arrow.
xNAP1L morpholino oligonucleotide blocks translation of xNAP1L RNA in vitro and in vivo. (A) Rabbit reticulocyte lysate was programmed with xNAP1L mRNA in the absence of MO (–mo) and in the presence of xNAP1L MO (NAP mo) and control MO (Con mo, the inverted NAP sequence); 35S-methionine–labeled proteins were separated by SDS-PAGE and visualized by autoradiography. No translation occurred in the absence of added RNA (–RNA). (B) To detect the effect of xNAP1L MO on xNAP1L protein levels in vivo, embryos were injected with 35S-methionine and xNAP1L mRNA (100 pg) to increase endogenous levels of the protein to detectable levels and either xNAP1L MO or control MO (12 ng). After immunoprecipitation with an xNAP1L polyclonal antibody, the radiolabeled zygotic protein was detected using a phosphorimager. To confirm the specificity of the antibody, an immunoprecipitation with preimmune serum (PI) was performed. The position of xNAP1L protein is shown by an arrow.
Blocking xNAP1L translation in embryos down-regulates α-globin mRNA
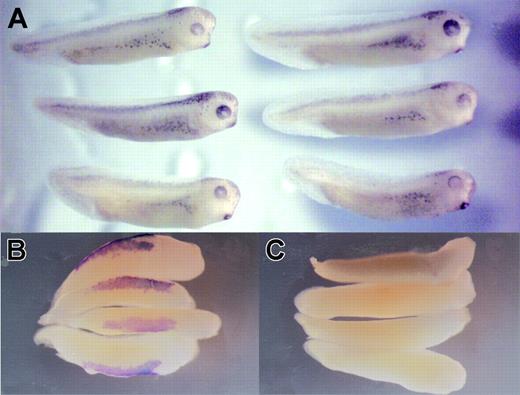
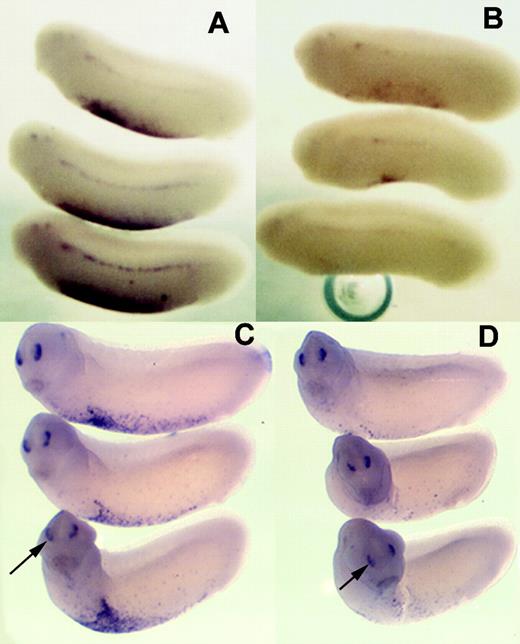
Embryos from which zygotic xNAP1L had been depleted appear morphologically normal (Figure 2A) and expression of a wide variety of marker genes (for example, cardiac actin, BMP-4, neural specific tubulin, and neural cell adhesion molecule (NCAM) assayed by RT-PCR or in situ hybridization) is unaffected by depletion. Their sibling embryos lacked globin mRNA as a control for successful depletion (data not shown). The observable phenotypic consequence of depletion is paralysis; staining neurons with an human natural killer 1 (HNK1) antibody at late stages of development suggests that this results from their disorganization (A.A.-D. and M.J.G., unpublished data, June 2002), and this phenotype is under investigation. In situ hybridization showed that α-globin mRNA was undetectable in the VBI of MO-injected embryos (460/473 embryos), while injection of a control MO left α-globin mRNA levels unaffected (312/315 embryos; compare Figure 2B and 2C). In order to test whether NAP depletion prevented only erythroid terminal differentiation or also affected hematopoietic precursors, we tested its effect on the expression of transcription factors known to be necessary for hematopoiesis, SCL and Xaml. The expression of both of these genes in the VBI was clearly repressed in xNAP1L-depleted tailbud stage embryos (63/63 for SCL and 43/47 for Xaml; compare Figure 3A with 3B, and 3C with 3D). SCL mRNA was also somewhat reduced in the DLP mesoderm where it is expressed in the adult blood precursors, a region expressing xNAP1L at low levels1 ; however, Xaml expression in the nasal placodes, where xNAP1L expression is not detected, was unaffected (arrowed in Figure 3C-D).
xNAP1L-depleted embryos are morphologically normal but lack globin expression. Eggs from a single female Xenopus were injected with xNAP1L MO or control MO (12 ng) and allowed to develop to stage 33. xNAP1L MO–injected embryos (A, right) do not differ morphologically from control MO–injected embryos (A, left). αT4-globin mRNA was detected by in situ hybridization38 in control embryos (B, ventral view) but not in xNAP1L MO–injected embryos (C, ventral view).
xNAP1L-depleted embryos are morphologically normal but lack globin expression. Eggs from a single female Xenopus were injected with xNAP1L MO or control MO (12 ng) and allowed to develop to stage 33. xNAP1L MO–injected embryos (A, right) do not differ morphologically from control MO–injected embryos (A, left). αT4-globin mRNA was detected by in situ hybridization38 in control embryos (B, ventral view) but not in xNAP1L MO–injected embryos (C, ventral view).
SCL and Xaml mRNA levels are reduced in xNAP1L MO–injected embryos. Eggs from a single female Xenopus were injected with xNAP1L MO or control MO (12 ng) and allowed to develop to stage 26. In situ hybridization of these embryos shows normal levels of SCL mRNA in the VBIs of control MO–injected embryos (A) and greatly reduced levels in embryos injected with xNAP1L MO (B). Similarly, Xaml expression in the VBI is not affected by control MO (C) but is reduced by injection of xNAP1L MO (D). Xaml mRNA in the nasal placodes is unaffected by xNAP1L MO injection (arrows in C-D).
SCL and Xaml mRNA levels are reduced in xNAP1L MO–injected embryos. Eggs from a single female Xenopus were injected with xNAP1L MO or control MO (12 ng) and allowed to develop to stage 26. In situ hybridization of these embryos shows normal levels of SCL mRNA in the VBIs of control MO–injected embryos (A) and greatly reduced levels in embryos injected with xNAP1L MO (B). Similarly, Xaml expression in the VBI is not affected by control MO (C) but is reduced by injection of xNAP1L MO (D). Xaml mRNA in the nasal placodes is unaffected by xNAP1L MO injection (arrows in C-D).
NAP MO does not affect endothelial-marker expression
XNAP1L is expressed in the ventral region posterior to the cement gland from at least stage 18,1 coincident with the early hemangioblasts,27 thus potentially functioning at early stages in blood formation. To address at which point in hematopoiesis the effects of xNAP1L depletion are first observed, we tested the expression of the 2 hematopoietic precursor markers, SCL and Xaml, at the earliest stage when they are detectable. Both of these markers are observed in the precursors of the anterior VBI just posterior to the cement gland from stage 17 onward (Figure 4).27 In MO-injected embryos, in situ hybridization showed a consistent pattern of reduced expression for both of these markers (41 of 44 embryos for SCL, 47 of 50 embryos for Xaml). Posterior to the cement gland, expression was seen only at the periphery of the region that normally expresses these genes (Figure 4). To test whether this gene-expression pattern in fact resulted from cell death or incorrect developmental programming of the hematopoietic precursors, we took advantage of the fact that these cells have recently been shown to express not only hematopoietic but also endothelial markers and thus most likely represent hemangioblasts, precursors of both blood and vasculature.27 Expression of the endothelial markers xHex and xFli1 is unaffected both in intensity and position by depletion of xNAP1L (54 of 54 embryos for xHex, 67 of 67 embryos for xFli1) (Figure 4).
xNAP1L depletion causes reduction of blood but not endothelial marker expression in stage-17 embryos. In all panels, control MO–injected embryos are on the left and xNAP1L MO–injected embryos, on the right. Embryos were injected with 12 ng of the appropriate MO at the single-cell stage and allowed to develop to stage 17 when they were fixed and marker-gene expression was analyzed by in situ hybridization of SCL, Xaml, xHex, or Fli1. Ventral views of embryos show expression in the precursors of the VBI, and Xaml expression in neurons is shown in the dorsal view.
xNAP1L depletion causes reduction of blood but not endothelial marker expression in stage-17 embryos. In all panels, control MO–injected embryos are on the left and xNAP1L MO–injected embryos, on the right. Embryos were injected with 12 ng of the appropriate MO at the single-cell stage and allowed to develop to stage 17 when they were fixed and marker-gene expression was analyzed by in situ hybridization of SCL, Xaml, xHex, or Fli1. Ventral views of embryos show expression in the precursors of the VBI, and Xaml expression in neurons is shown in the dorsal view.
Due to the difficulty of obtaining quantitative data from in situ hybridization, we tested the effect of depleting xNAP1L on the overall levels of the mRNAs of these markers using quantitative real-time RT-PCR (RT-qPCR). At stage 18, both SCL and Xaml mRNA levels are reduced, while mRNAs encoding the endothelial markers are even slightly increased (Figure 5). Much of the remaining expression of SCL and Xaml is likely to be due to neural expression of these markers, which is not down-regulated by xNAP1L depletion (Figure 4). The mRNA encoding the GATA-2 transcription factor, which is essential for hematopoiesis,32 behaves similarly to the endothelial marker mRNAs showing a slight increase in expression in xNAP1L-depleted embryos (Figure 5). The altered pattern of gene expression when xNAP1L is depleted is stable during hematopoiesis, since mRNA levels in dissected VBI regions from stage-26 embryos recapitulate the earlier result (Figure 5). However, the increased reduction in SCL and AML expression observed upon xNAP1L depletion in the dissected VBIs when compared with intact embryos further supports the existence of a neural contribution to the levels of these transcripts in the stage-18 assays. These data, together with the fact that Fli1 is unaffected by xNAP1L depletion at earlier stages, when it is normally expressed at the same time as the affected SCL and Xaml genes (Alde Ciau-Uitz and R.K.P., unpublished data, November 2003), confirm that the failures in hematopoiesis are not due to retardation of development by the MO, which is always a concern with this type of experiment. RT-qPCR data also confirm that globin mRNA production is greatly reduced by xNAP1L depletion.
Rescue of hematopoietic gene expression in hemangioblasts by replacing xNAP1L can be non–cell autonomous
To confirm that the hematopoietic precursor defect was a specific result of zygotic xNAP1L depletion in the embryos, we attempted to rescue SCL expression by reintroducing xNAP1L. Injection into embryos of a synthetic mRNA that could not bind the MO, but encoded xNAP1L, resulted in recovery of SCL expression in the hemangioblast region (Figure 6A). We also tested whether xNAP1L was needed in the mesoderm for expression of SCL or, as suggested by its expression pattern on the outer edge of the VBI,1 in adjacent ectodermal cells. Injection of xNAP1L mRNA into the ectoderm was more effective at rescuing SCL expression (85% of 63 embryos were rescued, Figure 6A) than was injection into cells that will form the mesoderm (16% of 38 embryos were rescued, data not shown). The xNAP1L mRNA levels injected in these rescue experiments were lower than those that increase globin mRNA expression and induce abnormal bending.41 Thus, they do not produce an overexpression phenotype.
Replacement of SCL in the VBI of xNAP1L-depleted embryos restores α-globin expression
SCL is the earliest component of the hematopoietic transcriptional network known to be affected by xNAP1L depletion.43 To test whether the effect on SCL was potentially the cause of subsequent hematopoietic failure, we attempted to rescue globin mRNA levels in xNAP1L morphant embryos by SCL expression. This was successful in 43% of 42 embryos (Figure 6B). The levels of injected SCL mRNA were below those needed to cause ectopic globin expression,34 and therefore represent rescue as opposed to overexpression.
Quantitative RT-PCR confirms that hematopoietic but not endothelial marker genes are affected by xNAP1L depletion. Sets of 20 embryos injected with either the control or xNAP1L MO (12 ng) were allowed to develop to either stage 18 or 26 when RNA was prepared (from dissected VBI regions at stage 26) and levels of the mRNAs shown were assayed by real-time RT-PCR using Taqman probes and ODC as a control mRNA for normalization. The level of each mRNA in the control MO–injected embryos was set to 100% (they did not differ from uninjected embryos); measurements were made in triplicate and varied by less than 5%. The experiment was performed 3 times with very similar results; those from a single experiment are shown with the error bars representing the extreme spread of results.
Quantitative RT-PCR confirms that hematopoietic but not endothelial marker genes are affected by xNAP1L depletion. Sets of 20 embryos injected with either the control or xNAP1L MO (12 ng) were allowed to develop to either stage 18 or 26 when RNA was prepared (from dissected VBI regions at stage 26) and levels of the mRNAs shown were assayed by real-time RT-PCR using Taqman probes and ODC as a control mRNA for normalization. The level of each mRNA in the control MO–injected embryos was set to 100% (they did not differ from uninjected embryos); measurements were made in triplicate and varied by less than 5%. The experiment was performed 3 times with very similar results; those from a single experiment are shown with the error bars representing the extreme spread of results.
Rescue of hematopoietic-marker expression. Sets of embryos were injected with either the control or xNAP1L MO (12 ng). Depleted embryos were subsequently injected at the 4-cell stage with 50 pg synthetic mRNA encoding SCL into the marginal zone or at the 8-cell stage into all 4 animal blastomeres with 25 pg synthetic mRNA encoding xNAP1L. The embryos injected with xNAP1L mRNA were allowed to develop until stage 18 and were then fixed and analyzed for SCL mRNA by in situ hybridization (A). The embryos injected with SCL mRNA were allowed to develop until stage 30 and then were fixed and analyzed for α-globin mRNA by in situ hybridization (B).
Rescue of hematopoietic-marker expression. Sets of embryos were injected with either the control or xNAP1L MO (12 ng). Depleted embryos were subsequently injected at the 4-cell stage with 50 pg synthetic mRNA encoding SCL into the marginal zone or at the 8-cell stage into all 4 animal blastomeres with 25 pg synthetic mRNA encoding xNAP1L. The embryos injected with xNAP1L mRNA were allowed to develop until stage 18 and were then fixed and analyzed for SCL mRNA by in situ hybridization (A). The embryos injected with SCL mRNA were allowed to develop until stage 30 and then were fixed and analyzed for α-globin mRNA by in situ hybridization (B).
Discussion
These data show that depletion of xNAP1L affects hematopoiesis. This correlates with the expression pattern of zygotic xNAP1L; the mRNA is detected in the region of the embryo posterior and ventral to the cement gland at neurula stages (where hematopoietic precursors are found27 ), and xNAP1L expression is strikingly coincident with the VBI later in development.1 XNAP1L is also expressed in neural tissue,1 and neural development is affected by depletion of the zygotic protein. By contrast, Xaml expression in the nasal placodes, in which xNAP1L mRNA is not detected, is unaffected by depletion. Therefore, the effects of interfering with xNAP1L expression are restricted to tissues where it is normally expressed. Further evidence that the phenotypic effects observed in xNAP1L-depleted embryos are a specific consequence of this depletion comes from the fact that replacement of xNAP1L in depleted embryos restores both SCL and Xaml (data not shown) mRNA levels to those of controls and the mobility of embryos. The depletion experiments that we have performed affect only zygotic xNAP1L, suggesting that maternal xNAP1L (which persists until swimming tadpole stages1 ) has properties that prevent it from substituting for the function of its zygotic counterpart in hematopoiesis. Consistent with this, analysis of xNAP1L-containing complexes by gel filtration revealed a single complex prior to zygotic gene activation, with a second appearing subsequently (M.J.G., unpublished data, August 2002).
It is clear that xNAP1L is first required for progression of the hematopoietic pathway at its earliest stages, prior to the appearance of SCL and Xaml mRNAs. The hemangioblasts failed to express these hematopoiesis-specific genes but were still detectable using endothelial markers, showing that this was not a result of cell death but of incorrect developmental programming of the blood precursors. The fact that the effect of xNAP1L depletion on globin mRNA levels is much more pronounced than it is on expression of hematopoietic precursor genes (a 95% decrease in globin mRNA levels compared with 60% for SCL/AML in dissected VBIs where there is no neural contribution to distort the comparison) suggests that xNAP1L may also be necessary for terminal differentiation of erythroid cells. This dual effect may be caused by a direct requirement for xNAP1L in 2 distinct processes, hematopoietic precursor formation and terminal differentiation, or by a requirement for xNAP1L in the activation of a single gene that is itself required for both processes. SCL is a strong candidate for a single target gene since it is needed for both hematopoietic precursor formation44,45 and terminal differentiation.35 GATA-2 and Fli1, which directly control SCL expression,46 are normally expressed in depleted embryos, while SCL itself is repressed. A likely explanation for the observed failure in hematopoiesis is therefore that xNAP1L is required, together with GATA-2 and Fli1, for SCL activation. Consistent with this hypothesis, replacement of SCL mRNA in xNAP1L-depleted embryos by injection restored α-globin expression, showing that no part of the hematopoietic pathway downstream of SCL is affected by xNAP1L depletion.
Overexpression of xNAP1L in ectodermal explants of Xenopus embryos was previously shown to increase GATA-2 mRNA levels,1 which is in apparent contrast to the effect of xNAP1L depletion on GATA-2 (a slight increase in expression) reported here. However, there are important differences between the experiments. First, the overexpression had no effect on GATA-2 mRNA levels at the developmental stages tested in the depleted embryos,1 and second, the former experiments analyzed ectodermal GATA-2 expression in isolation using an animal cap assay, while those reported here used whole embryos. GATA-2 is expressed in the neural ectoderm47 at the later stages when the overexpression effect was observed and has a role in interneuron formation48 ; our previous observations may relate to that role.
Comparing the expression of xNAP1L and globin in the VBI has shown that the former is expressed in both the outer cells of the VBI and the ectoderm overlying it, suggesting that its role could involve signaling to promote hematopoiesis,1 although there is nothing to suggest that NAP itself is a secreted protein. The rescue experiments described here support this hypothesis; xNAP1L expression in the ectoderm was sufficient to rescue SCL expression in the mesoderm of depleted embryos,27 thus the requirement for xNAP1L in hematopoiesis can be non–cell autonomous. The signaling molecules known to affect blood formation at these early stages of development include BMP-4, basic FGF (bFGF), Indian hedgehog, and vascular endothelial growth factor (VEGF)27,28,49-51 ; in particular, Xenopus SCL expression has been shown to be BMP-4 dependent.52 Although overall levels of BMP-4 mRNA were unchanged by xNAP1L depletion (A.A.-D., unpublished data, August 2002), subtle changes to its levels and expression remain a possibility, and whether any of the other signaling molecules requires xNAP1L for its expression is currently unknown.
While it is difficult to envisage how the histone chaperone role of NAPs could be involved in the phenotype of depleted embryos, recent evidence that NAPs can act as classical coactivators of transcription13-15 does suggest a molecular mechanism to explain our observations. The data presented are consistent with a hypothesis in which xNAP1L is required for the transcription of a signaling molecule that activates SCL expression. Whatever the precise mechanism underlying our observations, zygotic xNAP1L clearly has a narrowly defined role in gene expression, differentiation, and development. Therefore, this widely expressed family of proteins, which have previously been assigned quite general roles, can have specific functions. xNAP1L is not alone among chromatin modifiers in having a defined developmental role; Baf60c, part of the Baf60 chromatin remodeling complex, is required for normal heart morphogenesis and muscle differentiation.53 Specific developmental roles may turn out to be widespread among proteins involved in chromatin regulation.
Prepublished online as Blood First Edition Paper, April 5, 2005; DOI 10.1182/blood-2005-02-0598.
Supported by the Biotechnology and Biological Sciences Research Council (BBSRC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to Maggie Walmsley and Aldo Ciau-Uitz for useful discussions and to Garry Scarlett, Geoff Kneale, and Colin Sharpe for suggesting improvements to the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal