Abstract
In early pregnancy, human extravillous trophoblasts (EVTs) invade and remodel maternal arteries. We have previously demonstrated that CCR1 is expressed on perivascular/endovascular trophoblasts and that CCR1 ligands promote EVT migration. In this study, we examined the physiologic roles of platelet-derived chemoattractants on EVT invasion. By immunohistochemistry, maternal platelets were localized among endovascular trophoblasts within the lumen of spiral arteries. Extracellular matrices (ECMs) were also detected among endovascular trophoblasts and platelets, suggesting that the platelets in these arteries were activated by ECMs. In vitro, platelets attached to EVTs isolated from human villous explant cultures and expressed P-selectin on the cell surface. Platelets significantly enhanced migration of EVTs without affecting proliferation of EVTs or secretion of MMP-2 or MMP-9. The invasion-enhancing effect of platelet-derived culture medium on EVTs was neutralized by anti-CCR1 antibody. Heat treatment completely abrogated the invasion-promoting effects of platelet-derived culture medium, but charcoal stripping did not. Platelets also induced endovascular trophoblast-like morphologic changes and integrin α1 expression in EVTs during 48-hour culture. These findings suggest that maternal platelets activated in the spiral arteries can regulate trophoblastic vascular infiltration and differentiation by releasing various soluble factors.
Introduction
In the human placenta, cytotrophoblasts show 2 distinct patterns of differentiation. In floating villi, cytotrophoblasts differentiate into syncytiotrophoblasts and form the syncytial layer while, at villous-anchoring sites, cytotrophoblasts differentiate into extravillous trophoblasts (EVTs) and form the stratified structure called the cell column. After EVTs lose proliferative activity and acquire migratory activity in the cell column,1 the cells begin to migrate into the decidual tissue (interstitial trophoblasts) or toward maternal blood vessels. Interestingly, EVT migration is directed preferentially to the uterine spiral arteries.2 EVTs that migrate around the blood vessels (perivascular trophoblasts) destroy the muscular linings, and those that migrate along the vascular lumen (endovascular trophoblasts) replace the endothelium. Thus, the maternal arteries are remodeled into low-resistance tubes that are unable to constrict. This process ensures adequate placental perfusion and contributes to the successful establishment of pregnancy.3 In fact, insufficient physiological remodeling has been reported in cases of preeclampsia and intrauterine fetal growth retardation.4 In contrast to other organ constructions in the embryo and placenta, this extraembryonic tissue remodeling occurs in maternal tissues and requires both maternal and embryo-derived cells for cooperative tissue construction. From this perspective, this process is more complex than those involved in organ development during embryogenesis.
Vascular infiltration of EVTs at the implantation site is mainly observed in humans and primates.2 Therefore, analysis of the mechanism is difficult and has been poorly understood for a long time. The predominant EVT migration toward maternal arteries suggests that some factor(s) derived from the endothelium or blood constituents directs this migration. It has been proposed that the relatively high oxygen tension in maternal arteries promotes trophoblastic differentiation toward an invasive phenotype.5 This theory could explain, at least in part, why EVT invasion is directed toward the uterine arteries. However, some reports demonstrated that EVTs become more invasive under hypoxic conditions.6 Thus, hypotheses about the molecular mechanisms that regulate trophoblast invasion and uteroplacental artery remodeling remain controversial.7
We previously reported that trophoblasts acquire a chemokine receptor, CCR1, as they differentiate toward EVTs and that migration of EVTs is promoted by CCR1 ligands such as RANTES (regulated on activation, normal T cells expressed and secreted) and macrophage inflammatory protein-1α (MIP-1α).8 EVTs also decreased the expression of dipeptidyl peptidase IV/CD26, which can degrade chemokines including RANTES.9,10 Notably, perivascular/endovascular trophoblasts in the shallow sites expressed CCR1 more intensely than interstitial trophoblasts. CCR1 expression was specifically limited to the EVTs that migrated from the distal site of the cell column in the anchoring villi into spiral arteries through the shell that covered the peripheral sites of the maternal decidua.8 This suggests that chemokines are candidate factors supporting trophoblastic vascular infiltration. Therefore, we immunohistochemically examined possible production sites of CCR1 ligands in maternal tissues. Our immunohistochemical study, however, did not detect dominant localization of chemokine-expressing cells around/within the maternal vessels.8
Recently, human platelets have been shown to release several chemoattractants including chemokines.11 Because platelets lose their stored substances immediately after activation, it is no wonder that we could not detect immunoreactive chemokines in platelets at the fetomaternal interface. Moreover, platelet activation is known to occur near damaged blood vessels. Thus, we considered that platelets might be the sources of chemoattractants such as chemokines that support trophoblastic vascular infiltration. To investigate this possibility, we examined the immunohistochemical localization of platelets in frozen sections of early human placental tissues. In addition, we examined the effects of platelet-derived soluble factors on the invasion or differentiation of EVTs in vitro.
Materials and methods
Tissue samples
Human endometrial and placental tissues for immunohistochemistry were obtained from 8 therapeutic hysterectomies performed for cervical neoplasia or uterine myoma during the secretory phase (n = 3) and normal pregnancies (9 and 10 weeks of gestation; n = 5). Fresh tissues were embedded in optical cutting temperature (OCT) compound (Miles, Elkhart, IN), snap-frozen in liquid nitrogen, and stored at –80°C until use. Human placental tissues for chorionic villous explant cultures were aseptically obtained from legal abortions of normal pregnancies (6 to 9 weeks of gestation; n = 32). Gestational age was calculated from the date of the last menstrual period and, if necessary, was adjusted according to ultrasonic measurements of the gestational sac and fetal crown-rump length. Informed consent for use of these tissues was obtained from all donors according to the Declaration of Helsinki. Use of the materials was also approved by the Ethics Committee of Kyoto University Hospital.
Reagents and antibodies
An enzyme-linked immunosorbent assay (ELISA) kit to measure the concentration of human RANTES was obtained from Biosource International (Camarillo, CA). Function-perturbing mouse anti–human CCR1 monoclonal antibody (mAb) (clone 141-2) was purchased from MBL (Nagoya, Japan). Mouse anti–human integrin α1 mAb (clone FB12) and anti-integrin α5 mAb (clone SAM-1) were obtained from Chemicon (Temecula, CA) and Serotec (Oxford, United Kingdom), respectively. Mouse anti–human CD41 (glycoprotein IIb/IIIa [GPIIb/IIIa]) mAb (clone M148) and anti-CD45 mAb (clone BT1229) were purchased from Novocastra Laboratories (Newcastle, United Kingdom) and Dako (Glostrup, Denmark), respectively. Fluorescein isothiocyanate (FITC)–conjugated mouse anti–human CD41 (clone M148) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). FITC-conjugated and nonconjugated mouse anti–human P-selectin/CD62P (clone AK-4) mAbs were obtained from BD Biosciences–Pharmingen (Tokyo, Japan). Antihuman melanoma cell adhesion molecule/CD146 (MCAM/CD146) mouse mAb (clone S-Endo1, immunoglobulin G1 [IgG1] class; Alexis Biochemicals, San Diego, CA) was also used to detect EVTs. Mouse anti–human collagen type I mAb (clone I-8H5), anti–collagen type IV mAb (clone IV-3A9), and antifibronectin mAb (clone 96-21F2) were all purchased from Daiichi Fine Chemical (Takaoka, Japan). Mouse anti–human cytokeratin 7 mAb (clone OV-TL12/30) and FITC-conjugated mouse anti–human cytokeratin 7 mAb (clone LP5K) were obtained from Dako and Cymbus Biotechnology (Hants, United Kingdom), respectively. FITC-conjugated sheep anti–human von Willebrand factor polyclonal antibody (pAb) was obtained from Binding Site (Birmingham, United Kingdom). FITC-conjugated and nonconjugated mouse IgG1 (clone DAK-GO1) and IgG2b (clone DAK-GO9) for negative controls were all obtained from Dako. For the blocking antibody in double immunochemistry, antitrinitrophenyl (anti-TNP) mAb, an unrelated mouse mAb, was employed.12 For the secondary antibody, FITC-conjugated rabbit anti–mouse Ig pAb (Dako) or rhodamine-conjugated goat anti–mouse Ig pAb (Santa Cruz Biotechnology) was used.
Double immunohistochemistry
Sections of frozen placental tissues 7 μm thick were fixed with acetone and incubated with anti-CD41, anti-CD45, anti-CD62P, anti–collagen type I, anti–collagen type IV, antifibronectin, or isotype-matched control mAbs (10 μg/mL) for 1 hour. The sections were then incubated with rhodamine-conjugated goat anti–mouse Ig pAb (diluted 1:100) for 30 minutes and blocked with anti-TNP mAb (20 μg/mL) for 30 minutes. The sections were then incubated with FITC-conjugated mouse anti–human cytokeratin 7 mAb (diluted 1:10), FITC-conjugated mouse anti–human CD41 mAb (10 μg/mL), FITC-conjugated sheep anti–human von Willebrand factor pAb (diluted 1:100), or FITC-conjugated negative control antibodies for 1 hour. The stained sections were mounted with PermaFluor Mountant Medium (Shandon Immunon, Pittsburgh, PA) and examined under a confocal laser scanning microscope (LSM5 Pascal, ver 2.8; Carl Zeiss, Jena, Germany). Some sections were stained with hematoxylin and eosin (H&E).
Isolation and culture of human platelets and PBMCs
Human platelets and peripheral blood mononuclear cells (PBMCs) were isolated as described previously.13,14 Whole blood was obtained from healthy volunteers (female, 25 to 35 years old, n = 35), immediately mixed with 3.8% wt/vol trisodium citrate dihydrate (ratio of blood to citrate is 9:1) in polypropylene tubes, and centrifuged at 200g for 15 minutes at 22°C.
Platelet-rich plasma (PRP) was centrifuged after adding a mixture of 4.5% wt/vol citric acid and 6.6% wt/vol dextrose at 50 μL/mL PRP. The sedimented platelets were resuspended in RPMI containing 5.4 mM EDTA (ethylenediaminetetraacetic acid), stabilized for 10 minutes at room temperature, centrifuged at 980g for 10 minutes at 22°C, and then suspended in RPMI (2 × 108/mL).
The remaining blood samples containing PBMCs were diluted with RPMI and layered on lymphocyte separation medium (ICN Biomedicals, Aurora, OH) and centrifuged at 980g for 20 minutes at 4°C. PBMCs were collected from the interphase layer and then resuspended in RPMI (2 × 106/mL).
These platelets and PBMCs were cultured in collagen type I–coated 6-well plates (Iwaki, Chiba, Japan) for 24 hours to collect platelet-conditioned medium (CM) or PBMC-CM.
Human chorionic villous explant culture and isolation of EVTs
EVTs that grew from explanted human chorionic villi were isolated as previously described.8 Briefly, placental tissues were aseptically dissected to remove decidual tissues and fetal membrane. The remaining chorionic villi were minced into about 2 mm fragments, soaked in fetal calf serum (FCS) (Gibco, Grand Island, NY), and individually placed in 10-cm dishes coated with collagen type I (Iwaki). After 4 hours of incubation to allow the explants to adhere, 10 mL RPMI containing 10% FCS was gently added and the explants were incubated under standard conditions for an additional 48 hours. After the formation of cell sheets and the migration of spindle-shaped cells from the adherent villous tips were observed,8 the outgrown cells were dispersed with 0.05% trypsin (Difco, Detroit, MI)/0.05% EDTA solution, passed through a 40 μ–pore nylon cell strainer to remove chorionic villous parts, and replated in collagen type I–coated 6-well plates (Iwaki). After 4 hours of incubation, nonadherent cells and debris were removed by washing with RPMI. The cells that remained attached were defined as “isolated EVTs” and used for further experiments. More than 95% of the isolated cells were confirmed to be positive for both cytokeratin 7 and MCAM/CD146, a marker of EVTs.15,16 The rest were vimentin positive (villous stromal cells), and cells that were positive for CD45 (leukocytes) or von Willebrand factor (endothelial cells) were hardly detected.8
Long-term coculture of the isolated EVTs with platelets or PBMCs
The isolated EVTs (2 × 105 cells per 2 mL RPMI with 1% FCS) were plated in collagen type I–coated 6-well plates. PBMCs (2 × 106/mL) or platelets (2 × 108/mL) in RPMI were inoculated into the culture insert (Becton Dickinson, Franklin Lakes, NJ), which had a 0.4 μm–pore membrane filter that was precoated with collagen type I (1 μg/mL; Koken, Tokyo, Japan), and were cocultured with the isolated EVTs. After incubation for 48 hours, changes in EVTs were observed and analyzed by flow cytometry. For morphologic analysis, the isolated EVTs were plated in collagen type I–coated 24-well plates with the 0.4 μm–pore membrane culture insert in the presence or absence of PBMCs or platelets in triplicate and the calculated length-width ratio as described in “Cell attachment, cell elongation, cell proliferation, and apoptosis detection assays.” These experiments were repeated 5 times.
In some cases, the isolated EVTs were directly incubated with or without platelets in the absence of a culture insert. After 24-hour culture, EVTs were detached using scrapers and suspended in Hanks balanced salt solution (HBSS) (Gibco) containing 0.1% bovine serum albumin (BSA) and 0.1% NaN3 The sedimented cells were reacted with anti–human MCAM/CD146, anti–human CD41, or isotype-matched control mAbs for 30 minutes at 4°C and then with rhodamine-conjugated goat anti–mouse Ig pAb for 30 minutes and blocked with anti-TNP mAb for 30 minutes. The cells were then incubated with FITC-conjugated mouse anti–human CD41, CD62P, or control mAbs at 4°C for 30 minutes in the dark. The cells were washed in HBSS, resuspended in glycerin and phosphate-buffered saline (PBS) (1:1), and observed under a confocal laser scanning microscope (Carl Zeiss).
Flow cytometry
After trypsinization, EVTs (n = 5) that had been cultured with or without human PBMCs or platelets were suspended in HBSS (Gibco) containing 0.1% BSA and 0.1% NaN3 The precipitated cells were incubated with anti–human integrin α1, α5, or isotype-matched control mAbs (100 μg/mL, 10 μL) for 30 minutes at 4°C and then with FITC-conjugated rabbit anti–mouse Ig pAb at 4°C for 30 minutes in the dark. Cell surface labeling was analyzed by a FACSCalibur (Becton Dickinson). Differences between the mean intensity of integrin α1 or integrin α5 expression levels were analyzed by 2-tailed paired t test.
Matrigel invasion assay
Invasion assay was carried out as previously described.8 A 6.4-mm cell culture insert with an 8 μm–pore polyethylene terephthalate membrane filter (Becton Dickinson) was placed in collagen type I–coated 24-well plates (Iwaki). The lower surface of the membrane filter was precoated with diluted Matrigel (200 μg/mL; Becton Dickinson). Four sets of invasion assays were performed as follows.
First, 800 μL RPMI without cells (control) or platelets or PBMC suspension (2 × 108 platelets per milliliter and 2 × 106 PBMCs per milliliter in RPMI) was poured into the lower well. The isolated EVTs (2 × 104 cells per 200 μL RPMI with 5% FCS) were inoculated into the upper well. After 12 hours of incubation, the culture medium in the upper well was collected for gelatin zymography. The EVTs that remained on the upper surface of the filter were thoroughly removed. Cells that reached the lower surface were fixed with 100% methanol at –20°C for 5 minutes and immunostained with mouse anti–human cytokeratin 7 mAb followed by FITC-conjugated rabbit anti–mouse Ig pAb to visualize trophoblasts. The filters were mounted with Immunon and examined under a confocal laser scanning microscope. The numbers of cytokeratin 7–positive cells were counted for quantification using NIH Image 1.61.
Second, the lower well was filled with 800 μL RPMI without (control) or with 100, 200, or 400 μL platelet-CM or 400 μL PBMC-CM.
Third, platelet-CM was heat treated (incubated at 95°C for 1 hour) or charcoal stripped (incubated with 0.1 g/mL charcoal at 4°C for 24 hours), and the lower well was filled with 800 μL RPMI without (control) or with 400 μL of intact, heat-treated, or charcoal-stripped platelet-CM.
Fourth, isolated EVTs (2 × 104 cells per 200 μL RPMI with 5% FCS) were incubated at 4°C for 30 minutes with anti-CCR1 function-perturbing antibody (10 μg/mL) or isotype-matched control antibody (10 μg/mL). After preincubation, the cells were cultured in the upper well for 12 hours and Matrigel invasion toward the intact platelet-CM was compared.
These experiments were performed in duplicate, and the average was defined as the invading cell number. Each result was expressed as the percentage of invading cell number in the control (without coculture or additive). The experiment was repeated at least 3 times using EVTs that had been isolated from distinct chorionic explant cultures at different gestational ages (6 to 9 weeks). The differences were analyzed by 1-way analysis of variance (ANOVA) followed by Scheffé F test for multiple comparisons.
Cell attachment, cell elongation, cell proliferation, and apoptosis detection assays
Isolated EVTs (2 × 103 cells) were suspended in 100 μL RPMI plus 1% FCS that contained PBMC-CM, platelet-CM with or without anti-CCR1 mAb at the same concentration as that in the parallel invasion assays. These cells were cultured in each well of collagen type I–coated 96-well plates (Iwaki) under standard conditions in triplicate and observed under a phase-contrast microscope (IMT-2; Olympus, Tokyo, Japan) to evaluate cell morphologic changes. After 48 hours of incubation, morphologic changes were recorded by digital camera (Camedia C5050; Olympus, Tokyo, Japan) and cell elongation was analyzed by calculating the cell length-width ratio, which was defined as the value of the longest cell length divided by the vertical width of the longest one. For phase contrast microscopy, a CDPlan 20L objective lens was used; for fluorescence staining, Plan-Neofluor 10 ×/0.3 NA, 20 ×/0.5 NA, and 40 ×/0.75 Ph2 lenses (Carl Zeiss, Jena, Germany) were used. All images were processed using Adobe Acrobat software (San Jose, CA). The average length-width ratio in 30 cells in each well and the mean values of triplicate wells were calculated. The cell size (area) was also calculated using NIH Image 1.61 (http://rsb.info.nih.gov/nih-image). Differences among treatment groups in 5 independent experiments were analyzed by 1-way ANOVA followed by Scheffé F test for multiple comparisons.
In some wells of 96-well plates, the cells were allowed to attach to collagen type I for 30 minutes, washed once with RPMI, and the number of attached cells in each well assessed using the Premix WST-1 Cell Proliferation Assay System (Takara, Kusatsu, Japan) and ELISA plate reader (Molecular Devices, Menlo Park, CA) according to the manufacturer's instructions. In other wells of the 96-well plates, the cells were cultured for 12 hours, washed twice with RPMI, and the number of viable cells in each well assessed. Alternatively, the cell lysate was collected from each well, and the number of apoptotic cells was assessed using Cell Death Detection ELISA Plus (Roche Molecular Biochemicals, Mannheim, Germany) and ELISA plate reader according to the manufacturer's instructions. These experiments were repeated at least 3 times and performed in duplicate, and then the average was defined as the “attached cell number,” “viable cell number,” or “apoptotic cell number.” Each result was expressed as the percentage of the cell number in the control (without additive). The differences were analyzed by 1-way ANOVA followed by Scheffé F test for multiple comparisons.
Gelatin zymography
Gelatin zymography was performed as previously described.17 Twenty microliters of culture medium harvested from the upper well of the invasion assay was electrophoresed under nonreducing conditions in a 7.5% sodium dodecyl sulfate (SDS)–acrylamide gel containing 2 mg/mL gelatin (Difco). After electrophoresis, the gel was washed, incubated at 37°C overnight in buffer containing 150 mM NaCl, 5 mM CaCl2, 0.5 g Brij 35, 50 mM Tris (tris(hydroxymethyl)aminomethane) HCl, pH 7.6, and then stained with 01% wt/vol Coomassie brilliant blue R-250 (Wako, Osaka, Japan) in 30% vol/vol methanol, 10% vol/vol acetic acid for 60 minutes and destained in 30% vol/vol methanol, 10% vol/vol acetic acid. Semiquantification of the bands corresponding to 72-kDa and 92-kDa gelatinases was performed using NIH Image 1.61.
Results
Localization of platelets and leukocytes at human fetomaternal interface
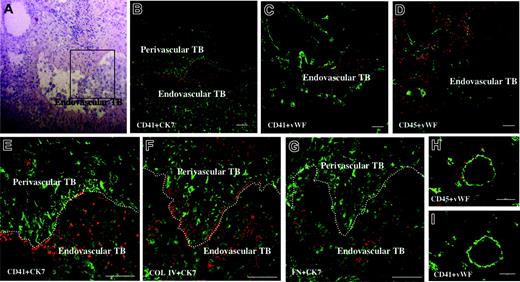
To determine the localization of platelets or leukocytes at the fetomaternal interface, we immunostained human placental tissues at 9 (n = 3) and 10 (n = 2) weeks of gestation with anti-CD41 mAb or anti-CD45 mAb, respectively. As shown in Figure 1B-D, platelets were confined within the maternal blood vessels that contained endovascular trophoblasts, while leukocytes were diffusely distributed throughout the maternal interstitium. These platelets were attached to the surface of the endovascular trophoblasts or to vessel walls that were infiltrated by perivascular trophoblasts (Figure 1E). Because it is well known that platelets become activated when they are attached to extracellular matrix (ECM), the expressions of collagen type I, collagen type IV, and fibronectin were examined in adjacent sections. Immunohistochemistry showed that all of these 3 ECM components were diffusely expressed in the maternal interstitium, including the subendothelial region. In addition, collagen type IV and fibronectin were expressed on the surface of endovascular trophoblasts in the spiral arteries (Figure 1F-G). Although CD45+ immune cells were distributed around spiral arteries, no platelets were observed within spiral arteries in the endometrium during the secretory phase in nonpregnant women (Figure 1H-I).
Colocalization of P-selectin and CD41 on platelets adhering to endovascular trophoblasts and cultured EVTs
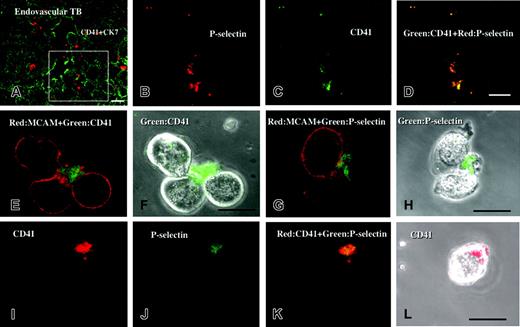
In the maternal spiral arteries, immunohistochemical double staining showed that P-selectin was colocalized with CD41 on platelets that adhered to endovascular trophoblasts (Figure 2A-D).
After 24-hour culture of isolated EVTs with platelets, immunocytochenical double staining showed that CD41+ platelets were adhered to CD146+ EVTs (Figure 2E-F). Most of these platelets expressed P-selectin on the cell surface, showing that they had been activated (Figure 2G-L).
Platelets are localized around the endovascular trophoblasts within the maternal vessels. Sections of placental tissue from therapeutic hysterectomy at 9 weeks of gestation were double stained with rhodamine (red) and FITC (green). (E-G) Higher magnifications of the boxed area indicated in panel A. (A) H&E staining. (B) CD41 (platelet marker, red) and cytokeratin 7 (CK7, marker for trophoblasts and endometrial gland, green). (C) CD41 (red) and von Willebrand factor (VWF, endothelial marker, green). (D) CD45 (leukocyte marker, red) and VWF (green). (E) CD41 (red) and CK7 (green). (F) Collagen type IV (COL IV, red) and CK7 (green). (G) Fibronectin (FN, red) and CK7 (green). TB, trophoblasts. Platelets were confined within the maternal blood vessel (C). These platelets were attached to the vessel wall (dotted white lines), which was infiltrated by spindle-shaped perivascular trophoblasts (E) or adhered to the surface of round endovascular trophoblasts (E), where collagen type IV and fibronectin were deposited (F-G). Endometrial sections from nonpregnant women in the secretory phase (postovulatory day 7) were also double stained by CD45 and CD41 (red; H and I, respectively) and VWF (green). CD45+ immune cells were distributed around spiral arteries, but no platelets were observed within the spiral arteries. Scale bars indicate 100 μm. Original magnification × 120 (panels A-D), × 320 (panels E-G), and × 200 (panels H and I).
Platelets are localized around the endovascular trophoblasts within the maternal vessels. Sections of placental tissue from therapeutic hysterectomy at 9 weeks of gestation were double stained with rhodamine (red) and FITC (green). (E-G) Higher magnifications of the boxed area indicated in panel A. (A) H&E staining. (B) CD41 (platelet marker, red) and cytokeratin 7 (CK7, marker for trophoblasts and endometrial gland, green). (C) CD41 (red) and von Willebrand factor (VWF, endothelial marker, green). (D) CD45 (leukocyte marker, red) and VWF (green). (E) CD41 (red) and CK7 (green). (F) Collagen type IV (COL IV, red) and CK7 (green). (G) Fibronectin (FN, red) and CK7 (green). TB, trophoblasts. Platelets were confined within the maternal blood vessel (C). These platelets were attached to the vessel wall (dotted white lines), which was infiltrated by spindle-shaped perivascular trophoblasts (E) or adhered to the surface of round endovascular trophoblasts (E), where collagen type IV and fibronectin were deposited (F-G). Endometrial sections from nonpregnant women in the secretory phase (postovulatory day 7) were also double stained by CD45 and CD41 (red; H and I, respectively) and VWF (green). CD45+ immune cells were distributed around spiral arteries, but no platelets were observed within the spiral arteries. Scale bars indicate 100 μm. Original magnification × 120 (panels A-D), × 320 (panels E-G), and × 200 (panels H and I).
Short-term effects of platelet- and PBMC-derived soluble factors on isolated human EVTs
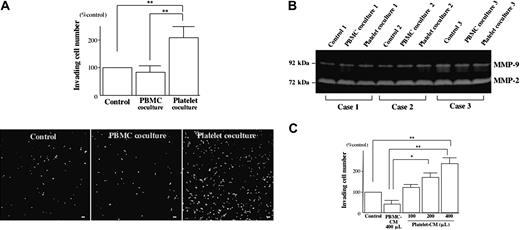
Coculturing with human platelets but not with PBMCs significantly enhanced Matrigel invasion of isolated human EVTs (Figure 3A) without affecting their secretion of matrix metalloproteinase-2 (MMP-2) or MMP-9 (Figure 3B). Similarly, platelet-CM enhanced the invasive activity of EVTs in a dose-dependent manner, while PBMC-CM did not (Figure 3C).
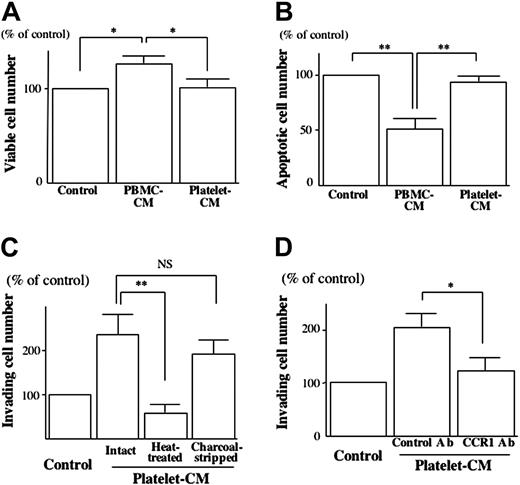
On collagen type I, neither platelet-CM nor PBMC-CM affected the attachment of EVTs (data not shown). After 12 hours of culturing on collagen type I, the number of viable cells (viability) was significantly increased (Figure 4A) and the number of apoptotic cells was significantly decreased in the presence of PBMC-CM (Figure 4B), but such effects were not observed with platelet-CM.
The invasion-promoting effect of platelet-CM on isolated EVTs was completely abrogated by heat treatment but not changed by charcoal stripping (Figure 4C). Interaction of chemokine receptor CCR1 and its ligands has been demonstrated to promote EVT invasion.8 To examine the possible contribution of CCR1 ligands to the invasion-promoting effect of platelet-CM, we disrupted the bioactivity of CCR1 on the isolated EVTs using an anti-CCR1 function-perturbing antibody. Preincubation of isolated EVTs with the anti-CCR1 antibody significantly reduced the invasion-promoting effect of platelet-CM (Figure 4D).
Long-term effects of platelet- and PBMC-derived soluble factors on the isolated human EVTs
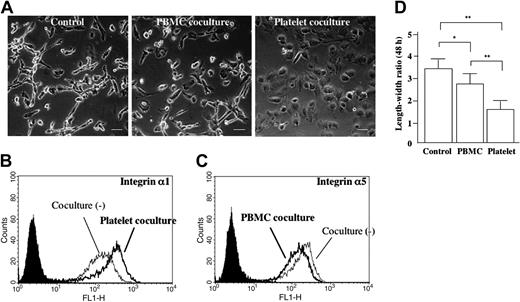
To examine the possible effects of PBMC- or platelet-derived soluble factors on EVT differentiation, isolated human EVTs were cocultured with human PBMCs or platelets for a longer period (48 hours). Without coculturing, the shape of isolated EVTs became extremely elongated after 48 hours, resembling that of interstitial trophoblasts in vivo (Figure 5A, left panel). The shape of isolated EVTs that had been cocultured with the platelets for 48 hours became rather round, resembling the shape of endovascular trophoblasts in vivo (Figure 5A, right panel). These morphologic changes were also confirmed by the length-width ratio (Figure 5D). There was no difference in cell size (data not shown). Flow cytometric analysis demonstrated that these round EVTs expressed higher levels of integrin α1 than control elongated EVTs (Figure 5B; mean relative intensity 180.543 ± 32.557 versus 122.817 ± 17.868, P < .05), while there was no significant change in integrin α5 expression (data not shown). When cocultured with PBMCs, integrin α1 expression was not changed (data not shown), but integrin α5 expression was significantly lower than that of controls (Figure 5C; mean relative intensity 242.438 ± 50.302 versus 284.140 ± 58.371, P < .05).
Colocalization of P-selectin and CD41 on platelets adhering to endovascular trophoblasts and cultured EVTs. Sections of placental tissue at 10 weeks of gestation were double stained with rhodamine (red) and FITC (green). Panels B-D are the sequential sections of panel A and higher magnifications of the boxed area indicated in the sequential section of panel A. P-selectin (B; red) was colocalized with CD-41 (C; green) on platelets that adhered to endovascular trophoblasts (A; green). After 24-hour culture of isolated EVTs with platelets, EVTs were detached from the dishes and were double stained. (F,H,L) Phase-contrast images. (E-F) CD41 (green) and CD146/MCAM (red). (G-H) CD62P/P-selectin (green) and CD146/MCAM (red). (I-L) CD62P/P-selectin (green) and CD41 (red). CD41+ platelets were adhered to CD146+ EVTs (E-F). Most of these platelets expressed P-selectin on the cell surface, showing that they had been activated (G-L). Bars show 20 μm. Original magnification × 400 (A), × 800 (panels B-D), and × 1200 (panels E-L).
Colocalization of P-selectin and CD41 on platelets adhering to endovascular trophoblasts and cultured EVTs. Sections of placental tissue at 10 weeks of gestation were double stained with rhodamine (red) and FITC (green). Panels B-D are the sequential sections of panel A and higher magnifications of the boxed area indicated in the sequential section of panel A. P-selectin (B; red) was colocalized with CD-41 (C; green) on platelets that adhered to endovascular trophoblasts (A; green). After 24-hour culture of isolated EVTs with platelets, EVTs were detached from the dishes and were double stained. (F,H,L) Phase-contrast images. (E-F) CD41 (green) and CD146/MCAM (red). (G-H) CD62P/P-selectin (green) and CD146/MCAM (red). (I-L) CD62P/P-selectin (green) and CD41 (red). CD41+ platelets were adhered to CD146+ EVTs (E-F). Most of these platelets expressed P-selectin on the cell surface, showing that they had been activated (G-L). Bars show 20 μm. Original magnification × 400 (A), × 800 (panels B-D), and × 1200 (panels E-L).
Platelet-derived soluble factors promote EVT migration. (A) Isolated human EVTs were allowed to invade through Matrigel toward human PBMCs or platelets that were cultured on collagen type I. After 12 hours, the EVTs that reached the lower surface were immunostained with cytokeratin 7 antibody (bottom) and counted for quantification using NIH Image 1.61 (top). Scale bars indicate 100 μm. Original magnification × 70. (B) At the end of the invasion assay, the culture medium was harvested from the upper well to evaluate the activity of MMP-2 and MMP-9 by gelatin zymography. (C) Matrigel invasion of isolated human EVTs toward PBMC-conditioned medium (CM) or platelet-CM was assessed by invasion assays.*P < .05; **P < .01.
Platelet-derived soluble factors promote EVT migration. (A) Isolated human EVTs were allowed to invade through Matrigel toward human PBMCs or platelets that were cultured on collagen type I. After 12 hours, the EVTs that reached the lower surface were immunostained with cytokeratin 7 antibody (bottom) and counted for quantification using NIH Image 1.61 (top). Scale bars indicate 100 μm. Original magnification × 70. (B) At the end of the invasion assay, the culture medium was harvested from the upper well to evaluate the activity of MMP-2 and MMP-9 by gelatin zymography. (C) Matrigel invasion of isolated human EVTs toward PBMC-conditioned medium (CM) or platelet-CM was assessed by invasion assays.*P < .05; **P < .01.
Analysis of the short-term effects of platelets on EVT function. (A-B) The isolated human EVTs were plated on collagen type I in the presence of PBMC-CM or platelet-CM. The viable cell number (A) and the apoptotic cell number (B) after 12 hours of culture were assessed as described in “Materials and methods.” The number of viable cells (viability) was significantly higher (A) and the number of apoptotic cells was significantly lower in the presence of PBMC-CM (B), but such effects were not observed with platelet-CM. (C-D) Matrigel invasion of isolated human EVTs toward platelet-CM with or without heat treatment or charcoal stripping in the presence or absence of anti-CCD1 mAb was assessed as described in “Materials and methods.” The invasion-promoting effect of platelet-CM on isolated EVTs was completely abrogated by heat treatment but not changed by charcoal stripping (C). Preincubation of isolated EVTs with the anti-CCR1 antibody significantly reduced the invasion-promoting effect of platelet-CM (D). *P < .05; **P < .01; NS, not significant.
Analysis of the short-term effects of platelets on EVT function. (A-B) The isolated human EVTs were plated on collagen type I in the presence of PBMC-CM or platelet-CM. The viable cell number (A) and the apoptotic cell number (B) after 12 hours of culture were assessed as described in “Materials and methods.” The number of viable cells (viability) was significantly higher (A) and the number of apoptotic cells was significantly lower in the presence of PBMC-CM (B), but such effects were not observed with platelet-CM. (C-D) Matrigel invasion of isolated human EVTs toward platelet-CM with or without heat treatment or charcoal stripping in the presence or absence of anti-CCD1 mAb was assessed as described in “Materials and methods.” The invasion-promoting effect of platelet-CM on isolated EVTs was completely abrogated by heat treatment but not changed by charcoal stripping (C). Preincubation of isolated EVTs with the anti-CCR1 antibody significantly reduced the invasion-promoting effect of platelet-CM (D). *P < .05; **P < .01; NS, not significant.
Discussion
In this study, we examined the localization of leukocytes and platelets in frozen sections of early human placental tissues. Immunohistochemistry showed that leukocytes (CD45+ cells) were diffusely distributed throughout the maternal interstitium. In contrast, platelets (CD41+ cells) were confined within maternal vessels containing endovascular trophoblasts. We could detect immunoreactive α-smooth muscle actin around these vessels, indicating that the vessels were arteries and not veins (data not shown). In spiral arteries of nonpregnant women, no platelets were detected. In pregnant women, these platelets were attached to the surface of endovascular trophoblasts or to vessel walls that were infiltrated by perivascular trophoblasts. The expression of ECM components such as collagen type IV and fibronectin was observed on the surface of endovascular trophoblasts as well as in the subendothelium. Considering that ECM can effectively trigger platelet activation,18 these platelets are likely to have been activated and to have released various bioactive substances. During activation, platelets transport P-selectin from the cytoplasmic region to cell surface. Therefore, to support the above-mentioned speculation, we confirmed that platelets that were directly incubated with isolated EVTs adhered to these EVTs and that the adherent platelets expressed P-selectin on their cell surface, showing that platelets have become activated.
Long-term coculturing with platelets induces EVT transformation. Human EVTs isolated at 6 weeks of gestation were cultured for 48 hours in the presence of human PBMCs or platelets that were plated in the collagen type I–coated upper chamber (A). Scale bars indicate 50 μm. Original magnification × 240. At the end of the coculture, EVTs were trypsinized and the cell surface expression of integrin α1 (B) and integrin α5 (C) was examined by flow cytometry. The EVTs became round (A, right), and their integrin α1 expression was enhanced in the presence of platelets (B). The filled curves are control mAb. These morphologic changes were also confirmed by length-width ratio (D). *P < .05; **P < .01.
Long-term coculturing with platelets induces EVT transformation. Human EVTs isolated at 6 weeks of gestation were cultured for 48 hours in the presence of human PBMCs or platelets that were plated in the collagen type I–coated upper chamber (A). Scale bars indicate 50 μm. Original magnification × 240. At the end of the coculture, EVTs were trypsinized and the cell surface expression of integrin α1 (B) and integrin α5 (C) was examined by flow cytometry. The EVTs became round (A, right), and their integrin α1 expression was enhanced in the presence of platelets (B). The filled curves are control mAb. These morphologic changes were also confirmed by length-width ratio (D). *P < .05; **P < .01.
On invasion assays, we found that platelets significantly increased the number of human EVTs invading through Matrigel without affecting proliferation of EVTs. A similar invasion-promoting effect was observed in CM harvested from human platelet cultures. In contrast to platelet-CM, PBMC-CM had no significant effect on invasion of EVTs. Although the number of viable cells was significantly higher, the number of apoptotic EVTs was significantly lower in the presence of PBMC-CM. In general, migrating EVTs have no proliferative activity.19 In addition, this culture system contained only 1% FCS to reduce the effects of other soluble factors in FCS, which is considerably lacking in survival factors. Thus, PBMC-CM is considered to exert some apoptosis-inhibiting effect on EVTs, suggesting that PBMCs prevent apoptosis of EVTs.
It is widely considered that EVTs undergo differentiation even after these cells enter the maternal tissue.20 The shape of endovascular trophoblasts seems to be round and distinct from the surrounding perivascular trophoblasts that are spindle shaped (Figure 1E-G). Moreover, endovascular trophoblasts reportedly express higher levels of integrin α1 than interstitial trophoblasts.21 Here, we found that isolated EVTs became round when cocultured with platelets for a longer period (48 hours). These cells differed from elongated EVTs observed after culturing without platelets. These round EVTs expressed higher levels of integrin α1 than the elongated EVTs. These findings suggest that platelet-derived soluble factors may induce EVT differentiation toward the endovascular phenotype.
Thrombophilic disorders are well known to be associated with recurrent fetal loss, preeclampsia, and intrauterine fetal growth retardation.22 Although platelet activation is considered nonspecific for EVTs, but a general phenomenon of ECM-secreting cells, according to past reports, trophoblasts abundantly express thrombomodulin23 and tissue- and urokinase-type plasminogen activators24-26 that can inhibit the coagulation pathway and affect the rapid degradation of fibrin at the trophoblastic surface. In preeclampsia and/or intrauterine fetal growth retardation, where trophoblasts have been reported to possess lower activity of plasminogen activators,27 the maternal spiral arteries exhibit acute necrotizing arteriopathy (acute atherosis) with frequent thrombosis.28 Therefore, it is possible that in normal pregnancy, intravascular thrombus formation may be prevented by perivascular/endovascular trophoblasts despite the presence of platelets that have been activated by ECM.
Our previous study demonstrated that EVTs express a chemokine receptor, CCR1, and that CCR1 ligands such as RANTES, MIP-1α, MCP-2, and hemofiltrate CC chemokine-1 (HCC-1) significantly enhanced the migration of EVTs using the same cell culture and invasion assay systems.8 In addition, it was reported that platelets contain MIP-1α and MCP-3, which are also CCR1 ligands.11 In the present study, pretreatment with anti-CCR1 function-perturbing antibody significantly inhibited the enhanced EVT migration toward platelet-CM, indicating that platelet-CM promotes EVT migration at least in part through the action of the chemokine-CCR1 system in vitro. Taken together with the fact that CCR1 expression was more intense on perivascular/endovascular trophoblasts as compared with interstitial trophoblasts,8 it is speculated that the interaction of trophoblastic CCR1 and chemokines released by activated maternal platelets plays an important role in trophoblastic arterial infiltration.
In this study, platelet-CM–stimulated EVT migration was not completely inhibited by CCR1 neutralization. These results suggest that some platelet-derived soluble factors other than chemokines are also responsible for the promotion of EVT migration. Previous studies by other investigators demonstrated that epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF), all of which are released from activated platelets,29 could enhance trophoblastic invasion.30,31 Recently, it was reported that activated platelets release abundant amounts of a lipid mediator, sphingosine-1-phosphate (S1P), which regulates the migration of various cell types.32 However, lipid removal from platelet-CM by charcoal stripping had no significant effect on its migration-promoting activity, while inactivation of bioactive peptides by heat treatment completely abrogated the activity. These findings suggest that some protein factors such as chemokines, not lipid factors, were due to the biologic effects of platelet-derived CM on EVT function observed in this study.
Long-term coculturing with platelets in vitro caused morphologic changes in EVTs consistent with their differentiation toward endovascular trophoblasts. Anti-CCR1 neutralizing antibody could not inhibit platelet-induced morphologic change (data not shown), suggesting that the factors that induce rounding of EVTs during long-term culture differ from those that promote EVT invasion. Further investigation to characterize individual factors derived from platelets would facilitate a clarification of mechanisms involved in trophoblastic migration as well as differentiation.
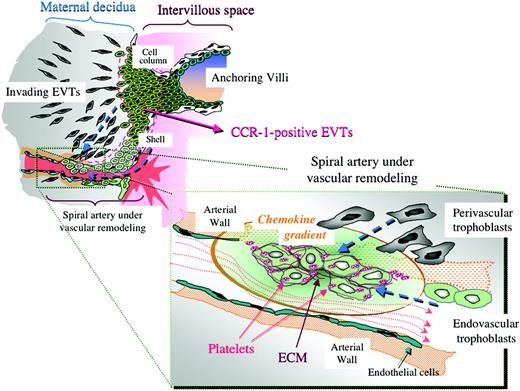
Possible chemokine gradient produced by platelets in spiral arteries and its estimated effects on trophoblast infiltration toward the arteries. In spiral arteries that are undergoing vascular remodeling, platelets and ECM are deposited among the endovascular trophoblasts. These platelets are likely to have been activated by trophoblasts or ECM and to have released various soluble factors including CCR1 ligands to form a local chemokine gradient around the remodeling spiral arteries. These processes may encourage further trophoblastic arterial infiltration and thus contribute to physiological vascular remodeling during human early pregnancy, providing a positive feedback cascade.
Possible chemokine gradient produced by platelets in spiral arteries and its estimated effects on trophoblast infiltration toward the arteries. In spiral arteries that are undergoing vascular remodeling, platelets and ECM are deposited among the endovascular trophoblasts. These platelets are likely to have been activated by trophoblasts or ECM and to have released various soluble factors including CCR1 ligands to form a local chemokine gradient around the remodeling spiral arteries. These processes may encourage further trophoblastic arterial infiltration and thus contribute to physiological vascular remodeling during human early pregnancy, providing a positive feedback cascade.
In summary, immunohistochemical examination confirmed the presence of platelets within the maternal spiral arteries at the fetomaternal interface, where these cells are likely to have been activated by ECM deposited around the endovascular trophoblasts and to have released various soluble factors. In vitro study confirmed that platelets that attached to EVTs isolated from human villous explant cultures were activated during the culture. This study also demonstrated that platelet-derived soluble factors promote the migration of EVTs, in part mediated by CCR1-chemokine system. In addition, platelet-derived soluble factors induce morphologic changes and integrin α1 expression of EVTs, compatible with their differentiation toward endovascular phenotypes. These findings suggest that activated maternal platelets within spiral arteries encourage trophoblastic arterial infiltration, thus contributing to physiological vascular remodeling (Figure 6). It was reported that essential thrombocythemia is related to recurrent abortion and fetal growth retardation, suggesting that platelets are deeply involved in early events during embryo implantation.33-35 The data presented here suggest the importance of platelet activation during maternal vascular remodeling, which is considered insufficient in such pathological pregnancies. Although antiplatelet (low-dose aspirin) therapy has been used worldwide for the prophylaxis of preeclampsia and/or intrauterine fetal growth retardation, there are many controversies about its effectiveness.36,37 Further investigation considering the physiologic roles of activated platelets in EVT function and/or differentiation will contribute to understanding the hematologic pathophysiology of recurrent abortion and preeclampsia/intrauterine fetal growth retardation in the future.
Prepublished online as Blood First Edition Paper, March 29, 2005; DOI 10.1182/blood-2005-02-0491.
Supported in part by Grants-in-Aid for Scientific Research (16390474 and 16659450).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Ms M. Takemura for technical assistance and to Dr Y. Ezumi and Dr T. Ishikawa in the Department of Hematology and Oncology in Kyoto University Hospital for valuable advice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal