Comment on Kopp et al, page 505
Hematopoietic recovery following chemoradiotherapy depends upon regeneration of the bone marrow microarchitecture; revascularization of bone marrow sinusoids with Tie2-expressing endothelial cells may constitute a defining event in this process.
The intimate association between hematopoietic and endothelial cells is both well established and incompletely defined. During early embryogenesis, the hematopoietic and endothelial lineages arise from a common precursor, the hemangioblast, and share expression of numerous “lineage-specific” molecules. In the adult, endothelial and hematopoietic cells play mutually supportive roles that are multifaceted. The study by Kopp and colleagues in this issue of Blood identifies one more facet, demonstrating that Tie2 signaling plays a key role in neovascularization of bone marrow (BM) sinusoids and consequent hematopoietic recovery following myelosuppressive treatment.FIG1
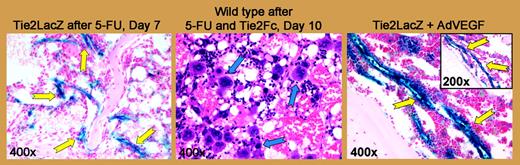
Vascular Tie2 expression is increased in a time-dependent manner after myelosuppression. See the complete figure in the article beginning on page 505.
Vascular Tie2 expression is increased in a time-dependent manner after myelosuppression. See the complete figure in the article beginning on page 505.
Within the heterogeneous architecture of the BM, distinct “niches” support different stages of hematopoiesis,1 from maintenance of hematopoietic stem cells (HSCs)/hematopoietic progenitor cells (HPCs) to expansion of progenitors, maturation of lineage-committed cells, and mobilization of mature cells to the circulation. Steady-state hematopoiesis requires the constant replenishment of mature blood cells, leading to a constant flux of cells between niches. In times of stress, the demands for rapid proliferation and maturation of appropriate hematopoietic lineages necessitate more dynamic changes within the marrow. Some of the most elusive aspects of hematopoietic regulation lie in the cellular and molecular signals that direct trafficking of HSCs and HPCs within the greater BM architecture and that support various aspects of hematopoiesis within distinct niches.
Using transgenic strategies and a model of chemotherapy-induced marrow injury, Kopp and colleagues provide new insights into the signals responsible for regeneration of the vascular niche and hematopoietic recovery in this setting. The data provide compelling evidence that Tie2 signaling is crucial for neovascularization and that interactions between hematopoietic and endothelial cells in the regenerating vascular niche promote thrombopoiesis. In the authors' model for hemangiogenic reconstitution, regression of BM sinusoidal vessels stimulates increased production of vascular endothelial growth factor A (VEGF-A), which up-regulates Tie2 expression on sinusoidal endothelial cells. The Tie2 ligand, angiopoietin-1 (Ang-1), further stimulates VEGF-A production and promotes proliferation and reassembly of the vascular architecture. It is interesting to note that Ang-1 also induces thrombopoiesis, even in thrombopoietin- and c-Mpl–null animals, perhaps by inducing migration and attachment of megakaryocytes to the neovasculature. Clearly, the physical association between megakaryocytes and regenerating vessels facilitates efficient production and release of mature platelets into the circulation; whether the cellular interactions between endothelial cells and hematopoietic progenitors also promote megakaryocyte maturation remains less clear.
As the authors suggest, it is intriguing to consider using angiogenic agents to stimulate recovery following myelosuppressive therapy in the clinical arena. However, some measure of caution may be warranted, as Ang-1–Tie2 signaling is not limited to the BM vascular niche and likely has context-dependent effects.2 While initially described as an “endothelial” receptor tyrosine kinase (RTK) having an essential role in angiogenesis and vascular remodeling, Tie2 is also expressed by HSCs and has recently emerged as a critical regulator of HSC maintenance in the osteoblastic BM niche.3 Furthermore, Ang-1 is also expressed by HSCs and HPCs, and these cells have been shown to promote migration and vascular remodeling by Tie2-expressing endothelial cells.4 These observations raise questions as to the precise role(s) of Ang-1–Tie2 signaling within the osteoblastic and vascular niches—and in the cellular migration between them. The integration of conserved signaling pathways, with RTKs providing “stop” and “go” signals for proliferation and differentiation of progenitors along specific lineage pathways, is a recurring theme in the regulation of developmental processes.5 If, in the BM, Tie2 provides a “stop” signal for HSCs in the osteoblastic niche and a “go” signal for endothelial cells in the vascular niche, stimulation of short-term revascularization and thrombopoiesis with Ang-1 might also delay long-term hematopoietic reconstitution. Thus, it might be wise to tie up some loose ends before embarking on a clinical path. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal