Epstein-Barr virus (EBV) establishes a latent, life-long infection in more than 95% of the human adult population. Antigen-specific, cytolytic T lymphocytes (CTLs) are thought to be of central importance in controlling EBV in both primary and latent infection states. While a good deal is known about the CTL dynamics during primary infection and convalescence,1-4 little is known about the CTL repertoire during the decades of infection after first contact. For example, do CTL clones persist indefinitely throughout an individual's lifetime or do new naive T-cell expansions emerge to help refresh the repertoire? We addressed this question by investigating the CTL dynamics toward immunodominant EBV epitopes up to 18 years apart, which is, to our knowledge, the longest follow-up study of T-cell responses conducted in humans.
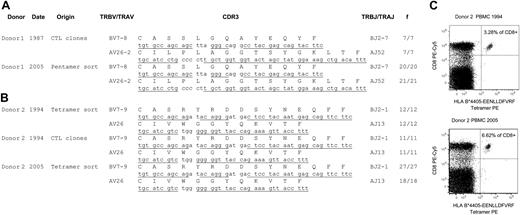
The CTL response toward a latent HLA B*0801-binding EBV epitope, FLRGRAYGL (FLR), has been described previously and was found to comprise large expansions of T cells bearing identical T-cell receptor (TcR) amino acid sequences in unrelated, B*0801+ individuals.5-8 In this follow-up study we investigated the FLR-specific T-cell repertoire of donor 1 using seven T-cell clones generated in 1987 and a pool of ex vivo, pentamer-sorted lymphocytes from 2005 (Figure 1A). Fifty-five TcR sequences derived from both time points revealed only a single TRBV7-8/TRAV26 clonal expansion, identical to the DNA level.
The CTL response toward another latent HLA B*4405-binding EBV epitope, EENLLDFVRF (EEN), has also been described previously,10 but no tetramer or TcR sequence information is currently available. As a result, we sequenced 91 TcRs from the EEN-specific CTL repertoire from donor 2 using a pool of ex vivo, tetramer-sorted T cells from cryogenically stored lymphocytes from 1994 and 2005 (Figure 1B). Like the FLR-specific repertoire in donor 1, the donor 2 EEN-specific repertoire consisted of a single, nucleotide-identical TRBV7-8/TRAV26 expansion. In addition, the clonal donor 2 EEN-specific expansion appeared to increase in frequency with time (Figure 1C). This observation in the donor, aged 61 years in 2005, is consistent with a previous study showing virus-specific CTLs sometimes increasing with age.11
Longitudinal analysis of the EBV-specific CTL response in the peripheral blood of two healthy donors. (A) V(D)J (variable-diversity-joining) junctional TcR sequences from FLRGRAYGL-reactive CTLs from donor 1 during 1987 and 2005, including CTL cell lines generated in 1987 and ex vivo HLA B*0801-FLRGRAYGL pentamer-sorted CD8+ cells in 2005. (B) V(D)J junctional TcR sequences from EENLLDFVRF-reactive CTLs from donor 2 during 1994 and 2005, including CTL cell lines generated in 1994 and ex vivo HLA B*4405-EENLLDFVRF tetramer-sorted CD8+ cells from 1994 and 2005. The TcR extraction procedure from CTL cell lines or pentamer/tetramer-sorted cells was performed as previously described.6,8 TcR germline sequences are underlined and TcR gene designations follow the nomenclature of the International ImMunoGeneTics Information System.9 (C) HLA B*4405-EENLLDFVRF tetramer-reactive T cells from donor 2 at the time points 1994 and 2005. Peripheral blood mononuclear cells (PBMCs) were stained with tetramer and anti-CD8 monoclonal antibody (mAb). The numbers in the right upper quadrants signify the frequency of CD8high cells that stained with tetramer. We sorted the tetramer-positive cells by fluorescence-activated cell sorting and performed a switch mechanism at the 5′ end of the RNA transcript (SMART) rapid amplification of complementary DNA ends (RACE) anchor polymerase chain reaction (PCR; Clontech, Mountain View, CA) on the extracted RNA. f values signify the frequency of TcR recovered from either CTL cell lines or multimer-sorted CD8+ T cells.
Longitudinal analysis of the EBV-specific CTL response in the peripheral blood of two healthy donors. (A) V(D)J (variable-diversity-joining) junctional TcR sequences from FLRGRAYGL-reactive CTLs from donor 1 during 1987 and 2005, including CTL cell lines generated in 1987 and ex vivo HLA B*0801-FLRGRAYGL pentamer-sorted CD8+ cells in 2005. (B) V(D)J junctional TcR sequences from EENLLDFVRF-reactive CTLs from donor 2 during 1994 and 2005, including CTL cell lines generated in 1994 and ex vivo HLA B*4405-EENLLDFVRF tetramer-sorted CD8+ cells from 1994 and 2005. The TcR extraction procedure from CTL cell lines or pentamer/tetramer-sorted cells was performed as previously described.6,8 TcR germline sequences are underlined and TcR gene designations follow the nomenclature of the International ImMunoGeneTics Information System.9 (C) HLA B*4405-EENLLDFVRF tetramer-reactive T cells from donor 2 at the time points 1994 and 2005. Peripheral blood mononuclear cells (PBMCs) were stained with tetramer and anti-CD8 monoclonal antibody (mAb). The numbers in the right upper quadrants signify the frequency of CD8high cells that stained with tetramer. We sorted the tetramer-positive cells by fluorescence-activated cell sorting and performed a switch mechanism at the 5′ end of the RNA transcript (SMART) rapid amplification of complementary DNA ends (RACE) anchor polymerase chain reaction (PCR; Clontech, Mountain View, CA) on the extracted RNA. f values signify the frequency of TcR recovered from either CTL cell lines or multimer-sorted CD8+ T cells.
This study follows the CTL response toward two immunodominant class I-binding EBV epitopes over a period of 18 or 11 years. Remarkably, we found no evidence of perturbations in the T-cell hierarchy over time, with both clonal CTL expansions remaining unchanged. Overall, the study provides an important advance in understanding the longevity and stability of the T-cell repertoire toward a persistent pathogen. Indeed, these findings suggest the likely situation of a single naive T-cell expansion facilitating the control of a persistent viral infection for the entire span of a human life.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal