Bone marrow (BM)-derived circulating endothelial precursor cells (CEPCs) have been reported to incorporate into newly formed blood vessels under physiologic and pathologic conditions. However, it is unknown if CEPCs contribute to lymphangiogenesis. Here we show that in a corneal lymphangiogenesis model of irradiated mice reconstituted with enhanced green fluorescent protein (EGFP)-positive donor bone marrow cells, CEPCs are present in the newly formed lymphatic vessels. Depletion of bone marrow cells by irradiation remarkably suppressed lymphangiogenesis in corneas implanted with fibroblast growth factor-2 (FGF-2). Further, transplantation of isolated EGFP-positive/vascular endothelial growth factor receptor-3-positive (EGFP+/VEGFR-3+) or EGFP+/VEGFR-2+ cell populations resulted in incorporation of EGFP+ cells into the newly formed lymphatic vessels. EGFP+/CEPCs were also present in peritumoral lymphatic vessels of a fibrosarcoma. These data suggest that BM-derived CEPCs may play a role in “lymphvasculogenesis.”
Introduction
Vasculogenesis is an important mechanism that contributes to blood vessel neovascularization. This process is not only essential for the establishment of the initial cardiovascular system during early embryonic development but also participates in pathologic neovascularization in the adult.1-3 For example, tumor angiogenesis recruits circulating endothelial precursor cells (CEPCs), and depletion of bone marrow (BM)-derived CEPCs retards tumor growth.4 Thus, inhibition of the recruitment of CEPCs to the newly formed tumor vessels may be an important approach for cancer therapy.
In addition to blood vessels, tumors are also able to stimulate the growth of lymphatic vessels, which can mediate lymphatic metastasis.5,6 Lymphatic vessel growth is regulated by several lymphangiogenic factors, including members of the vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and angiopoietin families.7-12 In the VEGF family, VEGF-C and VEGF-D have been considered as relatively specific lymphangiogenic factors due to their interactions with VEGF receptor-3 (VEGFR-3), a tyrosine kinase receptor specifically expressed on lymphatic endothelial cells (LECs). In addition to VEGF-C and -D, VEGF-A, also called vascular permeability factor, is able to stimulate lymphangiogenesis.13-15 Because VEGF-A is one of the most frequently overexpressed angiogenic factors found in various tumors, it might also be one of the key factors for tumor lymphangiogenesis. The mechanism by which VEGF-A induces lymphangiogenesis might involve both direct and indirect effects on LECs. VEGFR-2 has been detected on endothelial cells of the lymphatic vessels, suggesting its direct role in a stimulation of lymphangiogenesis.11,16-18 On the other hand, VEGF-A might recruit inflammatory cells that indirectly stimulate lymphangiogenesis through the secretion of cytokines and growth factors.15 Similarly, FGF-2-induced inflammation also significantly contributes to its lymphangiogenic activity in animal models.10
Sprouting of new lymphatics from the preexisting lymphatic vessels appears to be a critical mechanism for the formation of new lymphatic vessels in the adult.19 It is unclear if bone marrow (BM)-derived circulating endothelial cells participate in lymphangiogenesis. BM-derived CEPCs have the ability to differentiate into endothelial cells in vivo.20 These putative CEPCs have been defined as a cell population that expresses CD34 and VEGFR-2, 2 cell surface markers shared by embryonic endothelial progenitors and hematopoietic stem cells.21 Recently, VEGFR-3 and CD133/CD34+ cells were defined as a population of lymphatic/vascular precursor endothelial cells.21 However, it is not known if these cells are able to incorporate into the newly formed lymphatic vessels in vivo. In this report, we provide evidence that CEPCs are present in newly formed lymphatics of adult animals.
Materials and methods
Reagents and animals
Rat anti-mouse platelet endothelial cell adhesion molecule-1 (PECAM-1) (CD31) monoclonal antibodies and rat anti-mouse CD34 monoclonal antibodies were purchased from BD Pharmingen (San Diego, CA). VEGFR-3 antibodies were purchased from R&D Systems (Minneapolis, MN). VEGFR-2 antibodies were obtained from ImClone Systems (New York, NY). Guinea pig anti-mouse podoplanin antibodies, rat anti-mouse lymphatic vascular endothelial hyaluronan receptor-1 (LYVE-1) antibodies, and rabbit anti-mouse LYVE-1 antibodies were kindly provided by Dr David Jackson (The John Radcliffe Hospital, Oxford, United Kingdom). Female and male 6- to 8-week-old C57BL/6 mice were acclimated and caged in groups of 6 or less. Animals were anesthetized by intraperitoneal injection with a mixture of midazolam and fentanyl-fluanisone (1:1) before all procedures and killed by a lethal dose of CO2. All animal studies were reviewed and approved by the animal care and use committee of the Northern Stockholm Animal Board.
Bone marrow transplantation
Enhanced green fluorescent protein (EGFP)-positive mice 6 to 8 weeks old were used as donors of BM. Bone marrow cells (BMCs) were prepared according to procedures previously described.22 Briefly, BMCs were flushed out with RPMI 1640 medium from femurs and tibiae using 21-gauge needles. BMCs were collected, suspended in RPMI 1640 medium, filtered through a 100-μm cell strainer, and counted. Cells were harvested by centrifugation, resuspended with phosphate-buffered saline (PBS), and intravenously injected at a total number of 1 × 106 cells per 100 μL PBS into recipient syngenic C57BL/6 mice that had received a total body irradiation (TBI) of 9 Gy (900 rad) immediately before the transplantation.
Mouse corneal micropocket assay
The mouse corneal angiogenesis assay was performed as previously described.7 Briefly, angiogenic factors together with a slow release polymer were implanted into corneal micropockets of mice. In the experiments presented in Figures 1, 2, the mice were divided into 3 groups: (1) nonirradiated mice (n = 5); (2) mice irradiated 2 days prior to pellet implantation (n = 6); and (3) mice receiving BM transplants 6 weeks prior to pellet implantation (n = 11). In another experiment presented in Figures 3, 4, 5, 6, growth factor-containing micropellets were implanted 2 days after irradiation. The growth factor-implanted mice received transplants twice with fluorescence-activated cell sorter (FACS)-sorted cells at a density of 2.5 × 105 VEGFR-2/CD34+/+, VEGFR-3/CD34+/+, or VEGFR-3/VEGFR-2-/- cells at day 3 or 6 after pellet implantation. Corneal micropockets were created with a modified von Graefe cataract knife in the eyes of C57BL/6 mice. A micropellet (0.35 × 0.35 mm) of sucrose aluminum sulfate coated with hydron polymer (Interferon Sciences, New Brunswick, NJ) containing 80 ng FGF-2 was implanted into each corneal pocket. The pellet was positioned 1.2 to 1.4 mm from the corneal limbus. After implantation, erythromycin/ophthalmic ointment was applied to each eye. Eyes were examined by a slit-lamp biomicroscope on day 7 after the pellet implantation.
Tumor studies
EGFP+ C57BL/6 mice receiving BM transplants were used for tumor studies. A single cell suspension of approximately 1 × 106 wild-type (wt) T241 fibrosarcoma cells in a volume of 100 μL PBS was subcutaneously implanted in the middle dorsum of each animal. Primary tumors were measured at indicated time points, and 80% of tumor volumes were resected when they had reached the size of 1.2 cm in diameter. The residual tumors were continuously grown before removal at day 28 for histologic analysis of presence of lymphatic vessels.
Cell sorting
EGFP+ BMCs were collected as described under “Bone marrow transplantation” and used for isolation of VEGFR-2/CD34+/+, VEGFR-3/CD34+/+, and VEGFR-3/VEGFR-2-/- cells.21 Briefly, bone marrow was collected by centrifugation and resuspended in RPMI 1640 medium. The red blood cells were lysed with an ammonium chloride lysing reagent (BD Pharmingen). Cells were washed with 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and 0.5% bovine serum albumin (BSA) in PBS and then labeled with specific antibodies against VEGFR-2, VEGFR-3, or CD34. After centrifugation and washing, the cells were transferred to FACS tubes and resuspended in PBS with 0.1% goat serum. A FACS mounted with 3 beam lasers was used for the analysis and sorting (Becton Dickinson, San Diego, CA).
Whole-mount staining
Corneas and tumors were stained using our previously published whole-mount method.7 The following antibodies were used either alone or in combinations: rat anti-mouse CD31 antibodies, rabbit anti-mouse LYVE-1 antibodies, guinea pig anti-mouse podoplanin antibodies, rat anti-mouse CD34 antibodies, and rat anti-mouse VEGFR-3 antibodies. Stained samples were analyzed using a Zeiss LSM 510 confocal laser-scanning microscope (CLSM) (Carl Zeiss, Jena, Germany) and 3.2 SP2 Image Browser 5 LSM version 3.2 software (Carl Zeiss).
Immunofluorescent staining
Cryostat sections of corneal tissues (5 μm) were incubated with specific antibodies against LYVE-1, CD31, or CD34 for 2 hours at room temperature. Tissue sections were washed twice with PBS and incubated with cyanine 5 (Cy5)-labeled secondary antibodies for 30 minutes, washed in PBS, counterstained with propidium iodide (PI), mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and stored at 4°C until use. Stained samples were analyzed using CLSM.
Image analysis
Confocal images were analyzed using Image Browser 5 LSM, v3.2 (Carl Zeiss) by performing orthogonal sectioning, 3-dimensional projection analysis, and morphometric examination (length and density of lymphatic vessels). Numbers of incorporated EGFP+ cells in lymphatic vessels were counted using green pixels and blue vessels in Adobe Photoshop 7.0 program (Adobe Systems, San Jose, CA). Statistical analysis was performed using the 2-tailed Student t test for independent samples. Values of P below .05 were considered statistically significant.
Results
Bone marrow was involved in corneal lymphangiogenesis
To investigate whether BM-derived circulating cells are involved in lymphangiogenesis, we chose the mouse corneal lymphangiogenesis model in our study. Under physiologic conditions, the corneal tissue lacks both blood vessels and lymphatic vessels.7 At day 7 after implantation, FGF-2 stimulated growth of both blood vessels and lymphatic vessels in nonirradiated mice as revealed by CD31 and LYVE-1 staining (Figure 1A-C). The newly formed LYVE-1+ lymphatic structures were generally CD31- in contrast to the newly formed blood vessels, which stained strongly for CD31. This suggests that blood vessels and lymphatic vessels are completely different vascular structures. Morphologically, the newly formed lymphatic vessels consisted of large-diameter vascular structures that grew more slowly than blood vessels at this time point. In irradiated mice, significant suppression of both blood vessel and lymphatic vessel growth was observed in the cornea as measured by vessel length and area (P < .01) (Figure 1D-F,J,K). To further study the role of BM-derived cells in lymphangiogenesis, we reconstituted the BM of irradiated mice with EGFP+ BMCs from donor mice. The reconstitution of BM with EGFP+ cells largely restored both hemangiogenesis and lymphangiogenesis in the cornea (Figure 1G-K). These data suggest that the BM is an important organ for hemangiogenesis and lymphangiogenesis. Although the underlying mechanisms by which the BM contributes to blood angiogenesis and lymphangiogenesis need to be further elucidated, incorporation of circulating endothelial progenitor cells and bone marrow-derived inflammatory cells appears to be involved in both hemangiogenesis and lymphangiogenesis.
Bone marrow cells contribute to lymphangiogenesis. A pellet containing 80 ng FGF-2 was implanted into each cornea micropocket of nonirradiated (A-C), irradiated (D-F), and irradiated with EGFP+-transplanted BM (G-I) C57BL/6 mice. At day 7 of implantation, corneal tissues were stained with a combination of anti-CD31 antibodies (A,D,G) and anti-LYVE-1 antibodies (B,E,H). CD31 and LYVE-1+ signals of the same sections were further analyzed for overlapping signals by CLSM analysis (C,F,I). Images were captured using a Plan Neofluar 10 ×/0.3 NA objective lens. Quantification of length (J) and area (K) of LYVE-1+ vessels from 5 to 7 sections.
Bone marrow cells contribute to lymphangiogenesis. A pellet containing 80 ng FGF-2 was implanted into each cornea micropocket of nonirradiated (A-C), irradiated (D-F), and irradiated with EGFP+-transplanted BM (G-I) C57BL/6 mice. At day 7 of implantation, corneal tissues were stained with a combination of anti-CD31 antibodies (A,D,G) and anti-LYVE-1 antibodies (B,E,H). CD31 and LYVE-1+ signals of the same sections were further analyzed for overlapping signals by CLSM analysis (C,F,I). Images were captured using a Plan Neofluar 10 ×/0.3 NA objective lens. Quantification of length (J) and area (K) of LYVE-1+ vessels from 5 to 7 sections.
LYVE-1+ structures express other lymphatic markers
To determine whether LYVE-1+ lymph vessels also express other lymphatic markers, we detected the expression of podoplanin and VEGFR-3, 2 known specific markers for lymphatics.23 As expected, podoplanin and VEGFR-3 showed a staining pattern almost identical to LYVE-1 (Figure S1G-L, available on the Blood website; see the Supplemental Figure link at the top of the online article). In contrast, LYVE-1+ vessels completely lacked expression of CD34, a blood vessel-specific marker (Figure S1D-F). Interestingly, LYVE-1+ vessels also showed nonoverlapping staining patterns with CD31 (Figure S1A-C). These data validate previous findings showing that LYVE-1+ structures are lymphatic vessels that express several LEC markers. For convenience, we have used LYVE-1 as a lymphatic marker in our subsequent studies.
Incorporation of circulating BM-derived EGFP+ cells into the newly formed lymphatics
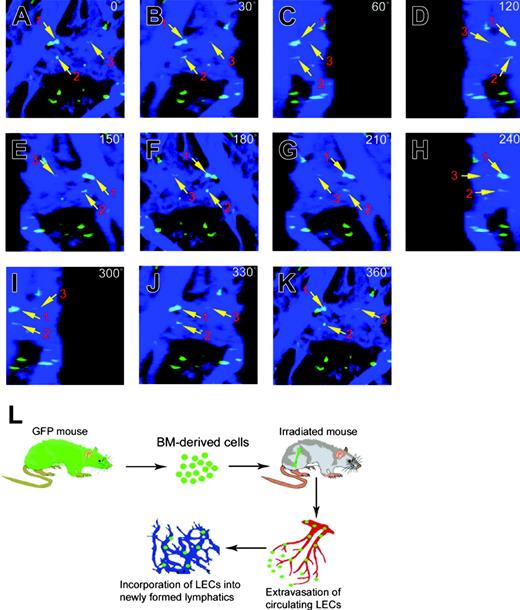
To study if BM-derived circulating cells contribute to lymphangiogenesis, we implanted FGF-2 pellets into mouse corneas of EGFP+ mice receiving BM transplants. At day 7, intensive neovascularization and lymphangiogenesis were detected in FGF-2-implanted corneas. Confocal microscopic analysis revealed that EGFP+ cells were indeed detectable in the LYVE-1+ vessels (Figure 2A, arrows). To determine whether these EGFP+ cells were incorporated into the wall of the lymphatics but not attached to the luminal side of lymphatic vessels, the relation between EGFP+ cells and lymphatic vessels was studied by 3-dimensional analysis. Figure 2 shows an example of 3 EGFP+ cells (marked with arrows and nos. 1 to 3) overlapping with LYVE-1+ structures, which were used for further analysis.
Confocal analysis using an orthogonal sectioning with a Zeiss LSM program at horizontal and vertical views revealed that EGFP+ cell no. 1 was indeed incorporated in the lymphatic vessel wall, whereas EGFP+ cell nos.2 and 3 were projected outside of the vessel wall (Figure 2B). Similarly, 3-D projection analysis of another region of the newly formed lymphatic vessels using a confocal program at different degrees showed that several EGFP+ cells, marked with nos. 1, 2, and 3, were incorporated in the lymphatic vessel wall (Figure 2C-M, arrows).
To validate these findings, a high-resolution microcopic method using thin tissue sections (5 μm) at high magnifications was applied for further studies. As shown in Figure 3A, some LYVE-1-positive structures showed overlapping staining signals with those of EGFP. To reveal the cellular identity, the vascularized corneal sections were further stained with PI, which stains cell nuclei (red). Interestingly, LYVE-1 and EGFP overlapping structures contained a cell nucleus, demonstrating that these structures were EGFP+ LECs (Figure 3A-D). Similarly, EGFP+ and VEGFR-3+ overlapping cellular structures were also present in the newly formed lymphatic vessels (Figure 3E-H). Morphologically, both LYVE-1+ and VEGFR-3+ structures exhibited thin walls and large flat lumens, which are considered to be the typical structural characteristics of lymphatic vessels as compared with CD31+, CD34+, or VEGFR-2+ structures (Figure 3C,G,K,O,S). In addition to incorporation into the newly formed lymphatic vessels, EGFP+ BMCs were also detectable in CD31-, CD34-, or VEGFR-2-expressing structures. (Figure 3I-T). These data further demonstrate that EGFP+ BMCs were present in newly formed lymphatic and blood vessels.
Presence of bone marrow-derived cells in newly formed corneal lymphatic vessels. Confocal microscopic analysis with high resolution was used to detect EGFP+ cells (green) in corneal lymphatic vessels (blue). EGFP and LYVE-1 overlapping positive signals were marked with numbers 1 to 3 (A,C-M, arrows). The EGFP+ cells were detected at different angles (0 degrees to 360 degrees [C-M]). The orthogonal sections show the relation of EGFP+ cells overlapping with LYVE-1 signals at the top, front, and side (B). Images were captured using a Plan Apochromat 20 ×/0.75 NA objective lens.
Presence of bone marrow-derived cells in newly formed corneal lymphatic vessels. Confocal microscopic analysis with high resolution was used to detect EGFP+ cells (green) in corneal lymphatic vessels (blue). EGFP and LYVE-1 overlapping positive signals were marked with numbers 1 to 3 (A,C-M, arrows). The EGFP+ cells were detected at different angles (0 degrees to 360 degrees [C-M]). The orthogonal sections show the relation of EGFP+ cells overlapping with LYVE-1 signals at the top, front, and side (B). Images were captured using a Plan Apochromat 20 ×/0.75 NA objective lens.
High-power microscopic analysis of the presence of EGFP+ cells in corneal vessels. Immunohistologically stained cryosections of FGF-2-implanted corneal tissues were analyzed by CLSM. EGFP+ signals were overlapped with LYVE-1 (B-D), VEGFR-3 (F-H), CD31 (J-L), CD34 (N-P), or VEGFR-2 (R-T). Tissue cell nuclei were counterstained with PI (red in panels A,E,I,M,Q). Arrows point to overlapping positive signals of EGFP and vessels. Images were captured with a 40 ×/1.30 NA Plan Neofluar objective lens.
High-power microscopic analysis of the presence of EGFP+ cells in corneal vessels. Immunohistologically stained cryosections of FGF-2-implanted corneal tissues were analyzed by CLSM. EGFP+ signals were overlapped with LYVE-1 (B-D), VEGFR-3 (F-H), CD31 (J-L), CD34 (N-P), or VEGFR-2 (R-T). Tissue cell nuclei were counterstained with PI (red in panels A,E,I,M,Q). Arrows point to overlapping positive signals of EGFP and vessels. Images were captured with a 40 ×/1.30 NA Plan Neofluar objective lens.
Presence of isolated EGFP/VEGFR-3+/+ or EGFP/VEGFR-2+/+ cells in the newly formed lymphatic vessels
To define what BM-derived cell populations contribute to lymphangiogenesis, we isolated VEGFR-2/CD34+/+ and VEGFR-3/CD34+/+ cell populations using FACS (Figure 4B-C). VEGFR-3/VEGFR-2-/- cells were collected and used as negative controls (Figure 4A). The same methodology and criteria as mentioned under “Incorporation of circulating BM-derived EGPC+ cells into the newly formed lymphatics” were used for assessment of incorporation of EGFP+ cells into the newly formed lymphatic vessels. Transplantation of these purified cell populations demonstrated that VEGFR-3+ cells significantly increased the degree of incorporation of EGFP+ cells into the FGF-2-induced newly formed lymphatics in the mouse cornea. Similarly, VEGFR-2+ cells were also found to significantly incorporate during active lymphangiogenesis (Figure 4D). In contrast, only few VEGFR-3/VEGFR-2-/- cells (less than 0.3%) were found in the vessel wall (Figure 4D). These data suggest that the BM-derived CEPCs were the primary cells contributing to lymphangiogenesis.
Immunohistologic analysis showed that VEGFR-3+/CD34+/EGFP+ cells were present in the thin wall of LYVE-1+ structures (Figure 4E-H). Similarly, VEGFR-2+/CD34+/EGFP+ cells were also found in the newly formed lymphatic structures (Figure 4I-L). In contrast, EGFP+ cells lacking VEGFR-3-and VEGFR-2 expression were rarely detectable in LYVE-1+ structures (Figure 4M-P). These findings provide evidence that the methodology for assessing the incorporation of EGFP+ cells used in our study seemed to be a valid approach, because only few VEGFR-3/VEGFR-2-/- cells were found in the vessel wall although the same number of cells was used.
Expression of endothelial cell markers in incorporated EGFP+ cells
To study if the EGFP+ cell populations incorporated in the newly formed lymphatics were of endothelial cell origin, we investigated the expression of several endothelial cell markers, including VEGFR-3, VEGFR-2, CD31, and CD34. Interestingly, isolated EGFP+ cells expressing VEGFR-3 or VEGFR-2 were indeed present in VEGFR-3+ or VEGFR-2+ vessel structures (Figure 5A-H,M-T). In contrast, VEGFR-3/VEGFR-2-/- cells were rarely detectable in these vascular structures (Figure 5I-L,U-X). In addition to lymphatic vessels, isolated VEGFR-2-expressing EGFP+ cell populations were detectable in CD31+ and CD34+ structures (Figure 6E-H,Q-T). Surprisingly, VEGFR-3-expressing EGFP+ cells were also found in CD31+ and CD34+ structures (Figure 6A-D,M-P). These findings suggest that VEGFR-3-positive cells derived from bone marrow might represent a population of precursor cells that can be incorporated into blood vessels or lymphatic vessels. We should emphasize that CD34/VEGFR-2+/+ or CD34/VEGFR-3+/+ cells might not represent clearly defined populations of double+ EPCs but could be a merely sort on the end of a long tail.
Transplantation of isolated BM VEGFR-2/CD34+/+ and VEGFR-3/CD34+/+ cells increased incorporation of EGFP+ cells in the newly formed LYVE-1+ structures. Bone marrow cells were FACS sorted for isolation of VEGFR-3/VEGFR-2-/- (A), VEGFR-2/CD34+/+ (B), or VEGFR-3/CD34+/+ (C) cells. Isolated cells were transplanted to irradiated mice that had been implanted with FGF-2 pellets in the corneas. The percentages of incorporated cells were counted in 5 random fields (D). Immunohistochemical analysis showing presence of VEGFR-3/CD34+/+ (E-H), VEGFR-2/CD34+/+ (I-L), or VEGFR-3/VEGFR-2-/- (M-P) cells within LYVE-1+ structures. Tissue cells nuclei were counterstained with PI (E,I,M). Arrows point to overlapping positive signals. Images were captured with a 40 ×/1.30 NA Plan Neofluar objective lens.
Transplantation of isolated BM VEGFR-2/CD34+/+ and VEGFR-3/CD34+/+ cells increased incorporation of EGFP+ cells in the newly formed LYVE-1+ structures. Bone marrow cells were FACS sorted for isolation of VEGFR-3/VEGFR-2-/- (A), VEGFR-2/CD34+/+ (B), or VEGFR-3/CD34+/+ (C) cells. Isolated cells were transplanted to irradiated mice that had been implanted with FGF-2 pellets in the corneas. The percentages of incorporated cells were counted in 5 random fields (D). Immunohistochemical analysis showing presence of VEGFR-3/CD34+/+ (E-H), VEGFR-2/CD34+/+ (I-L), or VEGFR-3/VEGFR-2-/- (M-P) cells within LYVE-1+ structures. Tissue cells nuclei were counterstained with PI (E,I,M). Arrows point to overlapping positive signals. Images were captured with a 40 ×/1.30 NA Plan Neofluar objective lens.
Presence of BM-derived EGFP+ cells in tumor lymphatics
To study if the BM-derived EGFP+ cells could also be detected in tumor lymphatics, murine T241 tumor cells were subcutaneously implanted on the back of EGFP+ C57BL/6 mice receiving BM transplants. At about 4 weeks after the implantation, relatively large tumors (about 1.2 cm3) were visible in mice. Tumors of this size were able to induce peritumoral lymphatics at the border region of the tumor tissues. Using the same analysis as for the corneal lymphangiogenesis system, we assessed the incorporation of EGFP+ cells in tumor lymphatics. Interestingly, EGFP+ cells were detected in the peritumoral lymphatic vessels (Figure 5A-K). These data suggest that BM-derived cells are likely to participate in tumoral lymphangiogenesis.
Incorporated EGFP+ BM cells express VEGFR-2 or VEGFR-3. Infusion of FACS-sorted VEGFR-3/CD34+/+ (A-D,M-P), VEGFR-2/CD34+/+ (E-H,Q-T), or VEGFR-3/VEGFR-2-/- (I-L,U-X) cells into irradiated mice. The FGF-2-induced vascular structures were immunohistologically analyzed for colocalization of double-positive signals of EGFP/VEGFR-3+/+ (A-L) and EGFP/VEGFR-2+/+ (M-X) cells. Arrows in panels D, H, L, P, T, and X point to overlapping signals. Images were captured with a 40 ×/1.30 NA Plan Neofluar objective lens.
Incorporated EGFP+ BM cells express VEGFR-2 or VEGFR-3. Infusion of FACS-sorted VEGFR-3/CD34+/+ (A-D,M-P), VEGFR-2/CD34+/+ (E-H,Q-T), or VEGFR-3/VEGFR-2-/- (I-L,U-X) cells into irradiated mice. The FGF-2-induced vascular structures were immunohistologically analyzed for colocalization of double-positive signals of EGFP/VEGFR-3+/+ (A-L) and EGFP/VEGFR-2+/+ (M-X) cells. Arrows in panels D, H, L, P, T, and X point to overlapping signals. Images were captured with a 40 ×/1.30 NA Plan Neofluar objective lens.
Incorporated EGFP+ BM cells express CD31 or CD34. Infusion of FACS-sorted VEGFR-3/CD34+/+ (A-D,M-P), VEGFR-2/CD34+/+ (E-H,Q-T), or VEGFR-3/VEGFR-2-/- (I-L,U-X) cells into irradiated mice. The FGF-2-induced vascular structures were immunohistologically analyzed for colocalization of double-positive signals of EGFP/CD31 (A-L) and EGFP/CD34 (M-X). Arrows in panels D, H, L, P, T, and X point to overlapping signals. Images were captured with a 40 ×/1.30 NA Plan Neofluar objective lens.
Incorporated EGFP+ BM cells express CD31 or CD34. Infusion of FACS-sorted VEGFR-3/CD34+/+ (A-D,M-P), VEGFR-2/CD34+/+ (E-H,Q-T), or VEGFR-3/VEGFR-2-/- (I-L,U-X) cells into irradiated mice. The FGF-2-induced vascular structures were immunohistologically analyzed for colocalization of double-positive signals of EGFP/CD31 (A-L) and EGFP/CD34 (M-X). Arrows in panels D, H, L, P, T, and X point to overlapping signals. Images were captured with a 40 ×/1.30 NA Plan Neofluar objective lens.
Discussion
The process of vasculogenesis usually refers to the de novo formation of blood vessels during early development of embryos. For example, the formation of the primitive heart and aorta during embryonic development is accomplished via the mechanism of vasculogenesis. Unlike the blood vessel system, the formation of the early lymphatic vessel in embryos occurs by differentiation of blood vessel endothelial cells into lymphatic endothelial cells.24,25 Sprouting of lymphatic capillaries from venous vessels is the mechanism for the formation of lymphatic vessels during embryogenesis. However, it is possible that “lymphvasculogenesis” may also contribute to the formation of lymphatic vessels in the early embryo. Because the lymphatic vessels usually lack hematopoietic cells inside the luminal structure, the stem cell-differentiated LECs are likely to be recruited from the environment of surrounding tissues. Similar to blood vasculogenesis, these lymphatic endothelial precursor cells (LEPCs) may be derived from BM, liver, and other hematopoietic organs. However, these LEPCs have to be extravagated from the blood circulation to contribute to lymphangiogenesis. Indeed, our bone marrow transplantation experiments suggest that EGFP+ BM-derived cells in the blood circulation were extravagated from blood vessels and subsequently incorporated into the newly formed lymphatic vessels (Figure 7L). However, it is unclear how LEPCs recognize the active lymphangiogenic sites for incorporation. Bone marrow-derived cells might be involved in lymphangiogenesis via 2 independent mechanisms: (1) differentiation of bone marrow stem cells into CEPCs participating in the formation of new lymphatics and (2) activation of bone marrow-derived inflammatory cells, which have been shown to significantly contribute to lymphangiogenesis by producing and secreting lymphangiogenic cytokines that induce lymphangiogenesis.15 Thus, irradiation of bone marrow might significantly impair lymphangiogenesis induced by inflammatory cells.
Incorporation of bone marrow-derived cells in tumor lymphatic vessels. High-resolution confocal microscopic analysis was used to show colocalization of EGFP (green) and LYVE-1 (blue) overlapping signals of lymphatic vessels at the surface of tumor tissues. EGFP+ cells overlapping with LYVE-1 signals were marked with numbers (1 to 3) and arrows (A-K). Schematic diagram demonstrating possible mechanisms of bone marrow-differentiated CEPCs in the participation of lymphangiogenesis. The bone marrow cells have to be extravagated from the blood vessels and incorporated into the newly formed lymphatics (L). Images in panels A-K were captured with a 10 ×/0.3 NA Plan Neofluar objective lens.
Incorporation of bone marrow-derived cells in tumor lymphatic vessels. High-resolution confocal microscopic analysis was used to show colocalization of EGFP (green) and LYVE-1 (blue) overlapping signals of lymphatic vessels at the surface of tumor tissues. EGFP+ cells overlapping with LYVE-1 signals were marked with numbers (1 to 3) and arrows (A-K). Schematic diagram demonstrating possible mechanisms of bone marrow-differentiated CEPCs in the participation of lymphangiogenesis. The bone marrow cells have to be extravagated from the blood vessels and incorporated into the newly formed lymphatics (L). Images in panels A-K were captured with a 10 ×/0.3 NA Plan Neofluar objective lens.
It appears that blood vessels and lymphatic vessels might use the same population of CEPCs for vasculogenesis and “lymphvasculogenesis.” It is known that BM-derived circulating VEGFR-2-positive cells significantly contribute to hemvasculogenesis under physiologic and pathologic conditions.1,8,14,26 Genetic studies in mice have shown that deletion of VEGFR-2 leads to a vascular defect where the blood islets are lacking, resulting in an impaired development of the hematopoietic and vascular systems.27 These results suggest that during early embryonic development VEGFR-2+ cells play a pivotal role for the differentiation into blood vessel endothelial cells and hematopoietic cells.28 Deletion of VEGFR-3 in mice results in defective sprouting of the lymphatics, including the sprouting of the initial lymphatic vessels from the embryonic veins.25 Therefore, it seems that VEGFR-2+ cells determine the early events of differentiation of embryonic stem cells into hematopoietic cells and vascular endothelial cells, whereas VEGFR-3+ cells control the development of the lymphatic system. In regard to BM cell populations, VEGFR-2+ BM cells mainly represent the circulating population of endothelial progenitor cells, and VEGFR-1-positive BM cells contribute to differentiation of nonendothelial cell lineages.29
It is known that tumor blood vessels recruit BM-derived VEGFR-2+ CEPCs for tumor angiogenesis.4 However, it is unclear whether VEGFR-2+ and VEGFR-3+ cells are involved in tumor lymphangiogenesis. Our present work suggests that VEGFR-2+ or VEGFR-3+ BM cells are involved in lymphangiogenesis. The CD34/VEGFR-2+/+ or CD34/VEGFR-3+/+ cells might not clearly define a distinct population of double-positive cells. We cannot exclude the fact that they might constitute a sorted population of less viable cells, because PI was not used for negative sorting of dead cells. Therefore, these data have limitations of interpretation. Thus, we should emphasize that incorporation of VEGFR-2+ or VEGFR-3+ cells in the newly formed lymphatics is suggestive but not conclusive. In this study, we further suggest that BM-derived cells might also be involved in tumor lymphangiogenesis. It is possible that the BM-derived cells can participate in lymphatic metastasis. Taken together, our findings might have therapeutic implications, and suppression of lymphvasculogenesis may be an important approach for prevention and treatment of lymphatic metastasis.
Prepublished online as Blood First Edition Paper, September 1, 2005; DOI 10.1182/blood-2005-01-0226.
Supported by research grants from the Swedish Research Council (Y.C.), the Swedish Heart and Lung Foundation, the Swedish Cancer Foundation, the Karolinska Institute Foundation, the Torsten and Rangar Foundation, the EU integrated projects of Angiotargeting (contract no. 504743), and VascuPlug (contract no. STRP 013811) networks.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 2. Presence of bone marrow-derived cells in newly formed corneal lymphatic vessels. Confocal microscopic analysis with high resolution was used to detect EGFP+ cells (green) in corneal lymphatic vessels (blue). EGFP and LYVE-1 overlapping positive signals were marked with numbers 1 to 3 (A,C-M, arrows). The EGFP+ cells were detected at different angles (0 degrees to 360 degrees [C-M]). The orthogonal sections show the relation of EGFP+ cells overlapping with LYVE-1 signals at the top, front, and side (B). Images were captured using a Plan Apochromat 20 ×/0.75 NA objective lens.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/13/10.1182_blood-2005-01-0226/2/m_zh80240587970002.jpeg?Expires=1767785687&Signature=SU3Xqm3XWw9InGD8LKCcDvpzHSHKtYMRhG8UTiQH3eMpg4a6Vp9OAGi0LmYCWv0IOMJbV7nLwcUoC2rzZ9xZIL~UHoszWsScaiGVfDtToxi6nDzQwMYyFXhpTuDOhnkclxRyQQn-9MdIlV~pfhid-WMu3P1J3GcXJJawXT2UHd0aSTC9-nmclnv0iKWT1goEVw8819xWmydebVZ~O6GKFsphEDdmuFfr8c~WvN9MZbEL7n3pY3~TfVEAT~z9JFjo~077X7Sjfsh-iqd5B9LnbQey0V2qges899iqfFlBsLsom892X--RNjsP4Yhh4G-SYBK2aeHri~suskG~xrzjDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal