Study objectives were to determine, in children with systemic lupus erythematosus (SLE), (1) the association of antiphosholipid antibody (APLA) subtypes with thrombotic events (TEs) and (2) the predictive value of persistent versus transient antibodies for TEs. This is a cohort study of 58 SLE children in whom lupus anticoagulants (LAs), anticardiolipin antibodies (ACLAs), anti–β2-glycoprotein-I (anti–β2-GPI), and antiprothrombin (anti-PT) were assessed on at least 2 occasions (more than 3 months apart). Antibodies were classified as persistent (positive on at least 2 occasions) or transient (positive once). Outcomes were symptomatic TEs confirmed by objective radiographic tests identified retrospectively and prospectively. Seven of the 58 patients (12%) had 10 TEs; 5 patients had TEs during prospective follow-up. Persistent LAs showed the strongest association with TEs (P < .001). Persistent ACLAs (P = .003) and anti–β2-GPI (P = .002) were significantly associated with TEs; anti-PT (P = .063) showed a trend. Persistent or transient LAs and anti–β2-GPI showed similar strength of association, while ACLAs and anti-PT were no longer associated with TEs. Positivity for multiple APLA subtypes showed stronger associations with TEs than for individual APLA subtypes because of improved specificity. Lupus anticoagulant is the strongest predictor of the risk of TEs; other APLA subtypes provide no additional diagnostic value. Anticardiolipin antibodies and anti-PT require serial testing because only persistent antibodies are associated with TEs.
Introduction
Antiphospholipid antibodies (APLAs) are a heterogeneous group of autoantibodies or alloantibodies directed at plasma protein/phospholipid complexes. Antiphospholipid antibodies frequently occur in autoimmune disease, such as systemic lupus erythematosus (SLE), and there is a well-documented association between the presence of APLAs and both venous and arterial thromboembolic events (TEs).1 Antiphospholipid antibodies also occur in infectious diseases, but these antibodies are usually not associated with TEs.2
There are 2 classic subtypes of APLAs: the lupus anticoagulants (LAs), which are detected by their interference with phospholipid-dependent coagulation tests, and anticardiolipin antibodies (ACLAs), which are quantitated by solid-phase immunoassays using phospholipids such as cardiolipin as antigen.3,4 Recently, immunoassays have been developed to specifically test for APLA subtypes directed at plasma protein cofactors binding to anionic phospholipids—most importantly, anti–β2-glycoprotein (anti–β2-GP-I) and antiprothrombin (anti-PT) antibodies.5,6 Assays for anti–β2-GP-I and anti-PT appear to be useful for discriminating APLAs found in autoimmune disease from postinfectious APLAs, while the conventional ACLA assay detects both types of antibodies.7-9
A considerable number of clinical studies have investigated the relationship of various APLA subtypes with the occurrence of TEs in diverse groups of patients (reviewed by Galli et al10 and Galli et al11 ). The results of these studies are controversial, with some studies suggesting anti–β2-GPI or anti-PT to be more closely associated with TEs and other studies showing no advantage over testing for LAs and ACLAs. There are several reasons for these inconsistencies between study results: First, all studies to date were of retrospective design and therefore were unable to assess the temporal relationship of presence of APLAs with the occurrence of TEs. There was variable selection of study populations, variable definition of clinical outcomes, and frequently lack of objective diagnosis of TEs. Second, there is inherent heterogeneity of APLAs and yet unresolved problems with assay standardization for both the classic assays for LAs and ACLAs as well as for anti–β2-GP-I or anti-PT.12-16 Third, studies did not discriminate between persistent and transient APLA subtypes, which is another means of discriminating pathogenic from benign APLAs.17,18
We have previously reported a highly significant association of presence of LAs with TEs in pediatric patients with SLE.19 The cohort consisted of nonselected children with SLE who had serial samples taken to test for APLAs and prospective outcome assessment for TEs. The objectives of the present study, performed in the same cohort, were (1) to compare the strengths of association of LAs, ACLAs, anti–β2-GP-I, and anti-PT with the occurrence of TEs and (2) to compare persistent versus transient APLA subtypes in their predictive value for the risk of TEs.
Patients, materials, and methods
Study design
The study design was an ambidirectional (retrospective and prospective) cohort study of nonselected children with SLE.
Study population
The study cohort overlaps with the cohorts of previously published studies.19-21 Patients were managed at the rheumatology clinics of 2 tertiary care centers: Hospital for Sick Children, Toronto, and Hôpital Ste Justine, Montreal. All patients fulfilled the American Rheumatism Association criteria for SLE.22 Consecutive children with SLE were recruited over a period of 2.8 years, except for 3 patients who did not consent. A disease activity index was determined at each study visit using a validated scoring system.23 A control population consisted of concurrent age-matched healthy children undergoing minor elective surgery. These children had an additional 5 mL of blood drawn at the time of venipuncture for preoperative blood work. Informed consent was obtained from all patients and control subjects or their guardians. The study was approved by both hospitals' institutional review boards.
Evaluation for thromboembolic events
All patients with SLE were followed prospectively for 4.5 to 7.5 years for clinically symptomatic TEs. All TEs were confirmed by objective radiographic tests. Additionally, all patients were assessed retrospectively for TEs that had occurred prior to study entry. Patients and/or their parents were interviewed by the nurse study coordinator, who was unaware of the laboratory results. A detailed history of previous TEs was obtained and a standardized questionnaire completed. A retrospective chart review was also performed and, if a TE was identified, the radiographic test that had been employed to confirm the TE was reviewed by an independent panel of experts.
Accepted radiographic tests for TEs were venography or ultrasound for deep venous thrombosis, a ventilation perfusion scan for pulmonary embolism, and either a computed tomography scan or magnetic resonance imaging for TEs in the central nervous system.
Laboratory testing
All study patients had blood samples taken at study entry and on a separate occasion during follow-up at least 3 months after the initial visit. Patients who developed an acute TE during the study period had blood drawn at the time of the event and a third time at least 3 months from the acute event. Patients in whom a blood sample was available from only one occasion were excluded (n = 5). Patients in whom the results of a specific APLA subtype were available from only one sample were not included in the analysis for the respective APLA subtype. Healthy controls had blood samples drawn on one occasion.
Venous blood was drawn into vacutainer tubes (Becton Dickinson, San Jose, CA) containing 0.105 M buffered citrate solution for a final ratio of 1 part anticoagulant to 9 parts blood. Plasma was immediately separated from cellular elements by double centrifugation at 1500g for 15 minutes at 4°C. Samples to be tested for LAs were filtered through a 0.2-μm screen (Acrodisk; Gelman Sciences, Ann Arbor, MI) to achieve complete platelet removal. Plasma was subsequently aliquotted and frozen at -70°C. All assays were performed in one central laboratory at the Pediatric Thrombosis Research Laboratory (Edmonton, AB, Canada) by the same technologist, who was blinded to the patients' clinical status. Lot numbers of all reagents were constant during the study.
Assays for lupus anticoagulants and anticardiolipin antibodies
Testing for LAs was performed according to the criteria of the Subcommittee on APLA of the International Society of Thrombosis and Haemostasis24 as previously described in detail19 : (1) screening assays included the kaolin clotting time, dilute activated partial thromboplastin time (aPTT), dilute prothrombin time, and 2 dilute Russel viper venom times; (2) all tests were repeated in a 1:1 mix with normal plasma; and (3) positive samples had confirmation assays performed with the same reagent in the presence of excess phospholipids. At least one test system had to be positive in all 3 steps for a patient to be considered LA positive.
ACLAs were measured by commercial enzyme-linked immunosorbent assay (ELISA) (Advanced Biological Products, Mississauga, ON, Canada). This assay is calibrated against standards from the Antiphospholipid Standardization Laboratory, University of Louisville, KY. Patient results were considered abnormal if values were 3 standard deviations above the mean of the age-matched controls.
Assay for anti–β2–glycoprotein-I antibodies
Anti–β2-GP-I was measured by in-house ELISA adapted from previously described methods.5,9,25-27 Polystyrene ELISA plates, γ-irradiated by the manufacturer (Immunolon-4, high-binding; Dynex, Chantilly, VA), were coated overnight at 4°C with 100 μL per well of purified human β2-GP (Affinity Biologicals, Hamilton, ON, Canada) in a concentration of 5 μg/mL in phosphate-buffered saline (PBS), pH 7.4 (8 g NaCl, 2.9 g Na2HPO4.12H2O, 0.2 KCL, 0.2 KH2PO4 per liter). Wells were blocked with 150 μL PBS containing 1% bovine serum albumin (PBS–1% BSA; BSA more than 99% purity; Sigma, St Louis, MO) for 1 hour at room temperature. Plates were then washed 4 times with PBS containing 0.1% Tween 20. Patient samples were diluted 1:100 in PBS–1% BSA–0.1% Tween 20, 100 μL applied to each well, and incubated for 2 hours at room temperature. Wells were then washed 4 times. Horseradish peroxidase–conjugated goat anti–human immunoglobulin G (IgG) (γ-chain specific; Sigma) and IgM (μ-chain specific; Sigma) were diluted in PBS–1% BSA–0.1% Tween 20 at 1:4000 and 1:6000, respectively, and 100 μL detecting antibody solution was applied to each well and incubated for 1 hour at room temperature. Plates were then washed 4 times. Color development was achieved by adding 5 mg ortho-phenylene-diamine to 12 mL citrate-phosphate buffer, pH 5.0, containing 0.03% H2O2 (vol/vol), 100 μL, to each well and incubated for 10 minutes. The reaction was stopped by the addition of 50 μL 2.5 M H2SO4 at timed intervals. Absorbances were read at 490 nm on a microplate reader (Spectra Max Plus 384; Molecular Devices, Sunnyvale, CA).
Assay for antiprothrombin antibodies
Anti-PT was measured by in-house ELISA adapted from previously described methods.6,27-30 The assay was performed as described for the assay for anti–β2-GPI with a few modifications: γ-irradiated polystyrene ELISA plates (Immunolon-4) were coated overnight at 4°C with 100 μL per well of purified human prothrombin (Enzyme Research Laboratories, South Bend, IN), 10 μg/mL in PBS, pH 7.4. Wells were blocked with 200 μL PBS containing 2% for 1 hour at room temperature and then washed 4 times with PBS–0.1% Tween 20. Patient plasma samples were diluted to 1:100 in PBS–1% BSA–0.1% Tween 20, 100 μL applied to each well in duplicate, and incubated for 2 hours at room temperature. After washing 4 times, horseradish peroxidase–conjugated goat anti–human IgG and IgM, 1:4000 in PBS–1% BSA–0.1% Tween 20, 100 μL per well, were incubated for 1 hour at room temperature. After washing 4 times, 5 mg ortho-phenylene-diamine in 12 mL citrate-phosphate buffer, pH 5.0, containing 0.03% H2O2 (vol/vol), 100 μL, was added to each well and incubated for 20 minutes. The reaction was stopped by the addition of 50 μL 2.5 M H2SO4 at timed intervals. Absorbances were read at 490 nm. Performing the assay in a PBS buffer system containing Ca++ (2 mM) gave similar readings as in the Ca++-free buffer in a series of positive and negative control samples.
For both anti–β2-GPI and anti-PT, noncoated wells were run on each plate and absorbances subtracted from antigen-coated wells to control for nonspecific binding. All experiments included controls of known samples with high, intermediate, and low antibody levels. For each APLA subtype and isotype, one positive control was chosen as internal standard, and the mean absorbance value measured for this standard was set at 100%. Absorbance values measured from unknown samples were expressed as percentage of the internal standard. For anti–β2-GPI IgG, external calibrators for anti–β2-GPI IgG were kindly provided by Dr V. DeBari, Paterson, NJ. Values obtained with these calibrators in our assay compared well with those reported by Dr DeBari's group,31 and an internal standard with comparable reactivity was chosen. Intraassay coefficients of variations for anti–β2-GPI IgG and IgM were 9.4% and 8.1%, and interassay coefficients of variations were 6.5% and 3.4%, respectively. Intra-assay coefficients of variations for anti-PT IgG and IgM were 10.8% and 9.2%, and interassay coefficients of variations were 5.8% and 4.5%, respectively.
Normal reference values were established by testing 20 healthy adults and 36 healthy children ages 1 to 18 years. Upper normal range cutoffs were derived from the 99th percentile of the reference population. Reference values for anti–β2-GPI IgG and IgM were slightly lower for children than for adults, and reference values for anti-PT IgG and IgM were higher for children than for adults. Within children, there were no differences between age groups for either antibody subtype or isotype. Cutoffs for children for anti–β2-GPI IgG and IgM were both 9% of the internal standard. Cutoffs for children for anti-PT IgG and IgM were 20% and 40%, respectively.
Classification of persistent versus transient positivity of antibodies
Patients were classified as “persistently positive” for an APLA subtype if positive on 2 or more occasions; “transiently positive” if positive on 1 occasion only; and negative if negative on all occasions.32,33 In the analysis, persistently positive patients were compared with all positive patients (ie, patients persistently or patients transiently positive) for an APLA subtype in their association with the occurrence of TEs.
Statistical analysis
Statistical analysis was performed using Minitab Statistical package, version 13 (Minitab, State College, PA), and SAS, version 8.2 (SAS Institute, Cary, NC). Values of antibody titers were log transformed because of skewed distributions. Antibody titers were compared between groups by t test (first versus follow-up sample). Repeated-measures analysis of variance was used to compare antibody titers between LA-positive and -negative and TE-positive and -negative patients and mixed models to assess correlations between individual antibody titers while accounting for repeat samples in each patient.
Frequencies of antibody positivity were analyzed in relation to frequencies of patients with or without TEs using contingency tables. Associations between antibody presence and occurrence of venous TEs were analyzed using the Fisher exact test and summarized by odds ratios (OR) and 95% confidence intervals (95% CI). Multivariable analyses to simultaneously test for the associations of several APLA subtypes with the absolute risk of venous TEs were performed using exact logistic regression. All tests were 2-sided.
Results
Study population
Fifty-eight children with SLE were studied: 48 females and 10 males. Their median age was 14.5 years with a range from 4 to 19 years. All but 2 patients with SLE were receiving corticosteroids during the study period. Oral contraceptives were taken by 2 patients who did not have a thrombotic event. There were no pregnancies. Disease activity scores were similar in patients with thrombotic events compared with other patients.19 The control population consisted of 36 concurrent healthy children: 18 females and 18 males. Their median age was 10 years with a range from 1 to 19 years.
Thrombotic events
Seven of the 58 patients with SLE (12%) had a total of 10 TEs. Three patients had TEs (n = 4) before they entered the study; 5 patients had TEs (n = 6) during prospective follow-up. Five events were deep venous TEs or pulmonary embolism, and 5 events involved the central nervous system, of which 3 events were sinovenous thromboses and 2 were arterial strokes.
All TEs were clinically symptomatic. All deep venous TEs were proximal in location, causing significant swelling and pain. The patient with pulmonary embolism presented with pleuritic chest pain and the ventilation perfusion scan that showed high probability of a pulmonary embolism. Central nervous system TEs caused a variety of neurologic symptoms depending on their location. There were no deaths. All TEs, including arterial strokes, were managed with heparin followed by warfarin for at least 3 months.
APLA subtype titers
Median time from initial to last follow-up blood samples was 5.5 months (minimum, 2.8 months; maximum, 34.5 months). In patients who had a TE identified retrospectively, the time from the TE to initial blood samples ranged from 3.4 to 46 months, and in patients with a TE identified prospectively, the time from the preceding sample to TE was a median of 10.2 months (minimum, 4.1 months; maximum, 46 months).
Titers of ACLA, anti–β2-GPI IgG, and anti-PT IgG did not differ in relation to time of sampling. Titers of anti–β2-GPI IgM showed a small decrease from initial to follow-up samples and anti-PT IgM a small increase (data not shown). Titers of ACLA, anti–β2-GPI, and anti-PT IgG and IgM showed no correlation with SLE disease activity scores (data not shown). APLA subtype titers were all significantly increased in LA-positive compared with LA-negative patients, accounting for repeat sampling in individual patients. There were significant correlations between most individual APLA subtypes, except for a lack of correlation between anti-PT IgG and the IgM of other subtypes and between anti-PT IgM and the IgG of other subtypes (data not shown).
Table 1 shows titers of APLA subtypes IgG and IgM comparing patients with and without TEs. ACLA, anti–β2-GPI, and anti-PT IgG titers were significantly increased in patients with TEs. Among patients with TEs, samples taken close to a TE tended to have increased titers of APLA subtypes compared with samples taken at other times, which reached significance for anti–β2-GPI IgM (median, 34% versus 18%; P = .05). In samples taken during warfarin treatment (n = 4), LA screening tests tended to have increased values compared with samples in patients with TEs when they were not on warfarin (n = 15). However, plasma mixing studies and confirmation tests were not different. All patients with TEs were classified as persistently positive for LAs in samples obtained on or off warfarin.
APLA subtype IgG and IgM titers in SLE patients with and without thrombotic events
. | Median antibody titers*(range) . | . | . | . | |
|---|---|---|---|---|---|
| APLA subtype . | Thombosis positive . | Thrombosis negative . | F value† . | P . | |
| ACLA IgG | 24.0 (4.5-65) | 11.0 (1.0-65) | 6.1 | .017 | |
| ACLA IgM | 31.0 (2.5-100) | 12.0 (2.5-100) | 6.1 | .017 | |
| Anti-β2-GPI IgG | 10.5 (2.7-91) | 3.5 (0-127) | 11.7 | .001 | |
| Anti-β2-GPI IgM | 21.0 (0.3-54) | 4.3 (0.4-78) | 3.9 | .054 | |
| Anti-PT IgG | 23.2 (5.9-135) | 11.7 (1.4-134) | 3.7 | .059 | |
| Anti-PT IgM | 22.0 (3.9-92) | 13.8 (1.2-111) | 0.6 | .453 | |
. | Median antibody titers*(range) . | . | . | . | |
|---|---|---|---|---|---|
| APLA subtype . | Thombosis positive . | Thrombosis negative . | F value† . | P . | |
| ACLA IgG | 24.0 (4.5-65) | 11.0 (1.0-65) | 6.1 | .017 | |
| ACLA IgM | 31.0 (2.5-100) | 12.0 (2.5-100) | 6.1 | .017 | |
| Anti-β2-GPI IgG | 10.5 (2.7-91) | 3.5 (0-127) | 11.7 | .001 | |
| Anti-β2-GPI IgM | 21.0 (0.3-54) | 4.3 (0.4-78) | 3.9 | .054 | |
| Anti-PT IgG | 23.2 (5.9-135) | 11.7 (1.4-134) | 3.7 | .059 | |
| Anti-PT IgM | 22.0 (3.9-92) | 13.8 (1.2-111) | 0.6 | .453 | |
For thrombosis-positive patients, n = 7; for thrombosis-negative patients, n = 51.
ACLA titers are given in IgG phospholipid (GPL) and IgM phospholipid (MPL) units based on standards from the Antiphospholipid Standardization Laboratory, University of Louisville, KY; anti-β2-GPI and anti-PT titers represent percentages of internal standards
The comparison is based on repeated-measures analysis-of-variance accounting for repeat sampling in each patient (initial and follow-up samples), performed on log-transformed values
Frequencies of APLA subtypes
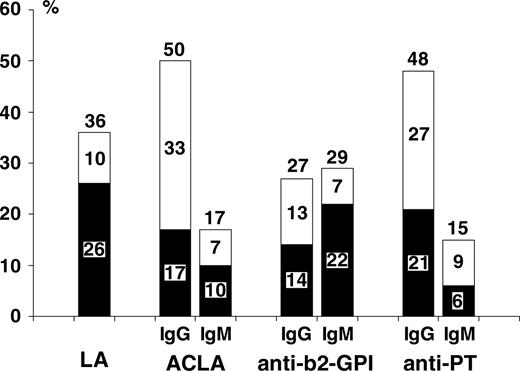
Figure 1 shows frequencies of presence of APLA subtypes, separate for IgG and IgM isotypes, in children with SLE. Frequencies are given for persistently positive antibodies, transiently positive antibodies, and all positive antibodies. LAs were persistent in 26%, transient in 10%, and persistent or transient in 36% of patients. ACLAs (IgG or IgM isotypes combined) were persistent in 21%, transient in 29%, and persistent or transient in 50% of patients. Anti–β2-GPI (IgG or IgM) was persistent in 31%, transient in 10%, and persistent or transient in 41% of patients. Anti-PT (IgG or IgM) was persistent in 27%, transient in 25%, and persistent or transient in 52% of patients.
Association of presence of APLA subtypes with thrombosis
Table 2 shows the association of presence of APLA subtypes (IgG or IgM combined) with the risk of TEs. Associations are compared for persistently positive antibodies versus persistently or transiently positive antibodies. Presence of LAs showed the strongest association with TEs, with an odds ratio that was infinite because no patient negative for LAs had a TE. Of the LA assays used, the dilute prothrombin time best predicted the risk of a TE, as previously reported in more detail.19 Persistently positive ACLAs and anti–β2-GPI were significantly associated with TEs, and there was borderline significance for anti-PT. Considering persistently or transiently positive antibodies, the strength of association remained about the same for LAs and anti–β2-GPI. In contrast, persistent or transient ACLAs and anti-PT were no longer significantly associated with TEs.
Association of presence of APLA subtypes, IgG or IgM combined, with thrombotic events; persistent versus persistent or transient antibodies
. | Persistent . | . | Persistent or transient . | . | ||
|---|---|---|---|---|---|---|
| APLA subtype . | OR (95% CI) . | P* . | OR (95% CI) . | P* . | ||
| Lupus anticoagulant | ∞ (6.0-∞) | < .001 | ∞ (3.2-∞) | < .001 | ||
| ACLA: IgG or IgM | 15.7 (2.5-97) | .003 | 2.8 (0.5-16) | .211 | ||
| Anti-β2-GPI: IgG or IgM | 22.0 (2.3-207) | .002 | 12.0 (1.3-110) | .014 | ||
| Anti-PT: IgG or IgM | 4.7 (0.9-25) | .063 | 2.6 (0.5-15) | .243 | ||
. | Persistent . | . | Persistent or transient . | . | ||
|---|---|---|---|---|---|---|
| APLA subtype . | OR (95% CI) . | P* . | OR (95% CI) . | P* . | ||
| Lupus anticoagulant | ∞ (6.0-∞) | < .001 | ∞ (3.2-∞) | < .001 | ||
| ACLA: IgG or IgM | 15.7 (2.5-97) | .003 | 2.8 (0.5-16) | .211 | ||
| Anti-β2-GPI: IgG or IgM | 22.0 (2.3-207) | .002 | 12.0 (1.3-110) | .014 | ||
| Anti-PT: IgG or IgM | 4.7 (0.9-25) | .063 | 2.6 (0.5-15) | .243 | ||
Fisher exact test
Table 3 shows sensitivity, specificity, and negative and positive predictive values of presence of APLA subtypes (IgG and IgM combined) for the risk of TEs. Persistently positive antibodies are compared with persistently or transiently positive antibodies. Presence of LAs had the highest predictive power for TEs, with a sensitivity and negative predictive value of 100%. The specificity for TEs was 84% for persistently positive LAs compared with 73% for all positive LAs. Persistently positive ACLAs and anti–β2-GPI had about equal predictive power. Considering persistent or transient antibodies decreased specificity and positive predictive value, particularly for ACLAs, because of a large proportion of transient ACLAs that were “false positive” (ie, not associated with a TE). Anti-PT had limited predictive value for the risk of TEs.
Predictive value of presence of APLA subtypes, IgG or IgM combined, for the risk of thrombotic events; persistent versus persistent or transient antibodies
APLA subtype . | Sensitivity, % . | Specificity, % . | NPV, % . | PPV, % . |
|---|---|---|---|---|
| Lupus anticoagulant | ||||
| Persistent | 100 | 84 | 100 | 47 |
| Persistent or transient | 100 | 73 | 100 | 33 |
| ACLA: IgG or IgM | ||||
| Persistent | 71 | 86 | 96 | 42 |
| Persistent or transient | 71 | 53 | 93 | 17 |
| Anti-β2-GPI: IgG or IgM | ||||
| Persistent | 86 | 79 | 97 | 40 |
| Persistent or transient | 86 | 67 | 97 | 30 |
| Anti-PT: IgG or IgM | ||||
| Persistent | 57 | 78 | 92 | 29 |
| Persistent or transient | 71 | 51 | 92 | 19 |
APLA subtype . | Sensitivity, % . | Specificity, % . | NPV, % . | PPV, % . |
|---|---|---|---|---|
| Lupus anticoagulant | ||||
| Persistent | 100 | 84 | 100 | 47 |
| Persistent or transient | 100 | 73 | 100 | 33 |
| ACLA: IgG or IgM | ||||
| Persistent | 71 | 86 | 96 | 42 |
| Persistent or transient | 71 | 53 | 93 | 17 |
| Anti-β2-GPI: IgG or IgM | ||||
| Persistent | 86 | 79 | 97 | 40 |
| Persistent or transient | 86 | 67 | 97 | 30 |
| Anti-PT: IgG or IgM | ||||
| Persistent | 57 | 78 | 92 | 29 |
| Persistent or transient | 71 | 51 | 92 | 19 |
NPV indicates negative predictive value; PPV, positive predictive value.
Frequencies of APLA subtypes IgG and IgM in children with SLE. Frequencies are shown for persistent (▪), transient (□), and all positive (persistent or transient) antibodies.
Frequencies of APLA subtypes IgG and IgM in children with SLE. Frequencies are shown for persistent (▪), transient (□), and all positive (persistent or transient) antibodies.
Table 4 shows the association of presence of APLA subtypes, separate for IgG and IgM isotypes, with the risk of a TE. Persistently positive antibodies are compared with persistently or transiently positive antibodies. The strongest association was observed for ACLA IgM and anti–β2-GPI IgG, with significant associations both for persistently positive and persistently or transiently positive antibodies due to high specificity. In contrast, only persistently positive ACLA IgGs were significantly associated with TEs. Persistently or transiently positive ACLA IgGs were not significantly associated with TEs because of low specificity (ie, a large proportion of “false-positive” transient ACLA IgGs). Anti-PT IgG and IgM showed no significant association with TEs.
Association of presence of APLA subtypes, separate for IgG and IgM isotypes, with thrombotic events; persistent versus persistent or transient antibodies
. | Persistent . | . | Persistent or transient . | . | ||
|---|---|---|---|---|---|---|
| APLA subtype . | OR (95% CI) . | P . | OR (95% CI) . | P . | ||
| ACLA IgG | 10.0 (1.8-56) | .013 | 2.8 (0.5-16) | .211 | ||
| ACLA IgM | 32.7 (4.2-256) | .001 | 10.0 (1.8-56) | .013 | ||
| Anti-β2-GPI IgG | 17.3 (2.6-116) | .005 | 10.6 (1.7-65) | .010 | ||
| Anti-β2-GPI IgM | 6.7 (1.2-37) | .036 | 9.2 (1.5-55) | .015 | ||
| Anti-PT IgG | 3.5 (0.7-19) | .155 | 3.1 (0.6-18) | .179 | ||
| Anti-PT IgM | 3.6 (0.3-46) | .358 | 2.6 (0.4-17) | .292 | ||
. | Persistent . | . | Persistent or transient . | . | ||
|---|---|---|---|---|---|---|
| APLA subtype . | OR (95% CI) . | P . | OR (95% CI) . | P . | ||
| ACLA IgG | 10.0 (1.8-56) | .013 | 2.8 (0.5-16) | .211 | ||
| ACLA IgM | 32.7 (4.2-256) | .001 | 10.0 (1.8-56) | .013 | ||
| Anti-β2-GPI IgG | 17.3 (2.6-116) | .005 | 10.6 (1.7-65) | .010 | ||
| Anti-β2-GPI IgM | 6.7 (1.2-37) | .036 | 9.2 (1.5-55) | .015 | ||
| Anti-PT IgG | 3.5 (0.7-19) | .155 | 3.1 (0.6-18) | .179 | ||
| Anti-PT IgM | 3.6 (0.3-46) | .358 | 2.6 (0.4-17) | .292 | ||
In multivariable analysis simultaneously testing for the associations of all individual APLA subtypes with TEs, LAs remained the only significant predictor of the risk of a TE.
Multiple positivity for 2 or more APLA subtypes did not improve the strength of association with TEs compared with LAs. However, multiple positivity for most APLA subtype combinations showed stronger associations with TEs than for individual ACLA, anti–β2-GPI, or anti-PT positivity because of improved specificity (Table 5).
Association of presence of multiple APLA subtypes with thrombotic events; persistent versus persistent or transient antibodies
. | Persistent . | . | Persistent or transient . | . | ||
|---|---|---|---|---|---|---|
| Combination of APLA subtypes: IgG or IgM . | OR (95% CI) . | P . | OR (95% CI) . | P . | ||
| LA + ACLA | 29.4 (4.3-203) | .001 | 15.7 (2.6-96) | .003 | ||
| LA + anti-β2-GPI | 40.0 (5.3-299) | < .001 | 55.2 (5.5-556) | .001 | ||
| LA + anti-PT | 37.5 (3.1-449) | < .001 | 15.7 (2.6-96) | .003 | ||
| ACLA + anti-β2-GPI | 40.0 (5.3-299) | < .001 | 23.0 (3.5-156) | .001 | ||
| ACLA + anti-PT | 15.7 (2.6-96) | .003 | 8.1 (1.4-47) | .020 | ||
| anti-β2-GPI + anti-PT | 21.3 (3.2-142) | .002 | 10.3 (1.7-61) | .010 | ||
| LA + ACLA + anti-β2-GPI | 32.7 (4.2-256) | .001 | 29.4 (4.3-203) | .001 | ||
| LA + ACLA + anti-PT | 37.5 (3.1-449) | < .001 | 29.4 (4.3-203) | .001 | ||
| LA + anti-β2-GPI + anti-PT | 37.5 (3.1-449) | < .001 | 40.0 (5.3-299) | < .001 | ||
| ACLA + anti-β2-GPI + anti-PT | 32.7 (4.2-256) | .001 | 29.4 (4.3-203) | .001 | ||
| LA + ACLA + anti-β2-GPI + anti-PT | 37.5 (3.1-449) | < .001 | 40.0 (5.3-299) | < .001 | ||
. | Persistent . | . | Persistent or transient . | . | ||
|---|---|---|---|---|---|---|
| Combination of APLA subtypes: IgG or IgM . | OR (95% CI) . | P . | OR (95% CI) . | P . | ||
| LA + ACLA | 29.4 (4.3-203) | .001 | 15.7 (2.6-96) | .003 | ||
| LA + anti-β2-GPI | 40.0 (5.3-299) | < .001 | 55.2 (5.5-556) | .001 | ||
| LA + anti-PT | 37.5 (3.1-449) | < .001 | 15.7 (2.6-96) | .003 | ||
| ACLA + anti-β2-GPI | 40.0 (5.3-299) | < .001 | 23.0 (3.5-156) | .001 | ||
| ACLA + anti-PT | 15.7 (2.6-96) | .003 | 8.1 (1.4-47) | .020 | ||
| anti-β2-GPI + anti-PT | 21.3 (3.2-142) | .002 | 10.3 (1.7-61) | .010 | ||
| LA + ACLA + anti-β2-GPI | 32.7 (4.2-256) | .001 | 29.4 (4.3-203) | .001 | ||
| LA + ACLA + anti-PT | 37.5 (3.1-449) | < .001 | 29.4 (4.3-203) | .001 | ||
| LA + anti-β2-GPI + anti-PT | 37.5 (3.1-449) | < .001 | 40.0 (5.3-299) | < .001 | ||
| ACLA + anti-β2-GPI + anti-PT | 32.7 (4.2-256) | .001 | 29.4 (4.3-203) | .001 | ||
| LA + ACLA + anti-β2-GPI + anti-PT | 37.5 (3.1-449) | < .001 | 40.0 (5.3-299) | < .001 | ||
Discussion
In patients with SLE, there is a significant association between the presence of APLAs and both venous and arterial TEs.1 However, APLAs are a heterogeneous group of antibodies, and not all patients with APLAs develop TEs. Therefore, there is a need to identify APLA subtypes that best predict the risk of TEs. Assays for APLA subtypes directed at specific plasma protein cofactors have been developed (ie, anti–β2-GPI and anti-PT), but the literature thus far is controversial as to whether these assays provide advantages over the classic assays for LAs and ACLAs. Moreover, there is no information as to whether the persistent presence of APLA subtypes strengthens the association with TEs.
The present study was performed in a nonselected cohort of pediatric SLE patients who had serial testing of APLA subtypes and prospective assessment for TEs, which were confirmed by objective tests. The study results demonstrate that (1) LAs were the strongest predictor of the risk of TEs and the only factor remaining significant in multivariable analysis and (2) persistent ACLAs and anti–β2-GPI were significantly associated with the risk of TEs, and anti-PT showed borderline significance. Considering persistently or transiently positive antibodies did not change the strength of association for LAs and anti–β2-GPI, but ACLAs and anti-PT were no longer associated with TEs.
The findings of the current pediatric study with respect to LAs are consistent with those of 2 systematic reviews of the literature. The first was a meta-analysis including 18 studies in adult patients with SLE demonstrating that LA shows stronger association with TEs than ACLAs.1 A more recent systematic review included 25 studies in adult patients with or without SLE.10 The review confirmed LAs as a strong risk factor for TEs but did not find unequivocal evidence for a significant association of ACLAs with TEs. In the present pediatric study, the association of LAs with TEs was even stronger than that reported for adults. The high negative predictive value (100%) for LAs reflects that no children negative for LAs had TEs. In contrast, of adult SLE patients negative for APLAs, approximately 40% had TEs.32 Therefore, when studying the association of APLA subtypes with TEs, a pediatric cohort is advantageous, because other confounding risk factors of TEs are rare in children, such as contraceptive use, pregnancy, cancer, artherosclerotic disease, and age. Because there was no comparison with adult SLE patients in this study, the effect of such risk factors could not directly be assessed. The specificity (84%) of LAs for the risk of TEs seen in the present study was also higher than reported in previous studies in adults.
Another recent systematic review summarized studies available to date (n = 32) on anti–β2-GPI and/or anti-PT and their association with clinical complications.11 The main message from that review is that there is considerable heterogeneity in designs of previous studies that did not allow a formal meta-analysis. All studies to date on anti–β2-GPI or anti-PT have been retrospective with the potential of selection in patient accrual, information bias in the assessment of clinical outcome, and the inability to assess a temporal relationship of presence of APLAs with the occurrence of TEs. Studies had variable definition of study populations, including patients with SLE, patients with primary antiphospholipid syndrome, or patients selected based on APLA positivity. Moreover, clinical outcomes were poorly defined and frequently not restricted to TEs, and many studies lacked objective diagnosis of TEs. Not surprisingly, the systematic review revealed large heterogeneity in the results of previous studies, with inconsistent information comparing the relative strength of associations of various APLA subtypes with TEs. Only a few studies have performed multivariable analysis to assess the joint contribution of individual APLA subtypes for prediction of the risk of TEs.
Our discussion of previous studies comparing several APLA subtypes will be restricted to studies that investigated SLE patients only, in nonselected cohorts, and focused on TEs as clinical outcomes.27,30,34-38 In these studies, each of LAs, ACLAs, anti–β2-GPI, and anti-PT were in most instances associated with TEs in univariate analysis. Multivariable analysis, performed in only part of studies, revealed inconsistent results, showing LAs only, anti–β2-GPI only, or LAs in combination with ACLAs or anti-PT significantly associated with TEs.27,36-38 The relative strengths of associations varied depending on antibody isotype and whether arterial or venous TEs were considered. However, there was no consistent association between a specific APLA subtype or isotype and either an arterial or venous TE. One pediatric study assessed LAs, ACLAs, and anti–β2-GPI in a heterogeneous cohort of patients with SLE, SLE-like syndrome, primary antiphospholipid antibody syndrome, or APLA positivity.39 The study did not find an association of any APLA subtype with TEs either in the total cohort or in the subgroup of children with SLE.
The main advantage of the present study over any previous study on anti–β2-GPI and anti-PT is its ambidirectional study design. Most patients (71%) had a TE identified during prospective follow-up, allowing the establishment of a temporal relationship with presence of APLAs. Each of the APLA subtypes were demonstrated to be associated with TEs. Anti–β2-GPI and persistent ACLAs showed about equal strength of association with TEs. In multivariable analysis, LAs remained the only significant risk factor for TEs. The functional assays for LAs may best reflect the pathogenic interference of APLAs with the coagulation system. The APLA subtypes tested by immunoassays had no additional diagnostic value to LAs. However, immunoassays for APLA subtypes may still be important in instances when coagulation assays for LAs are not feasible, such as in patients receiving oral anticoagulants. In this situation, ACLA IgM and anti–β2-GPI IgG have the best predictive value because of their high specificity.
The second advantage of the present study was that repeat samples were obtained from all patients, allowing the comparison of persistent versus transient APLA subtypes in their predictive value for the risk of TEs. According to current consensus, the antiphospholipid antibody syndrome is defined by the persistent presence of APLAs in combination with specific clinical complications such as TEs.40 Persistent APLA positivity is defined as the presence of the antibodies on 2 occasions at least 6 weeks apart. Persistent APLAs are considered to reflect a chronic autoimmune process and are more likely associated with TEs. Transient APLAs either reflect low disease activity or represent postinfectious antibodies and are frequently not associated with TEs. Particularly in children, frequent infections may trigger transient APLAs that rarely lead to clinical complications.41 The improved predictive value of persistent APLAs has thus far been shown for only LAs and ACLAs.17,18,32,33 Studies on anti–β2-GPI or anti-PT to date have not provided data to discriminate persistent from transient antibodies. Only one study reported repeat testing of anti–β2-GPI in SLE patients, but all anti–β2-GPIs observed in that study were persistent.42
In the present study, persistent LAs, ACLAs, anti–β2-GPI, and anti-PT were all associated with the risk of TEs. Considering persistently or transiently positive antibodies decreased specificity, but the overall strength of association with TEs remained about the same for LAs and anti–β2-GPI. In contrast, ACLAs and anti-PT were no longer associated with TEs when transient antibodies were included. Thus, anti–β2-GPI is advantageous over ACLAs, because a single positive test is associated with TEs. ACLAs and anti-PT require repeat testing because only persistent antibodies are significantly associated with TEs.
Another interesting finding of the study was that positivity for multiple APLA subtypes showed stronger associations with TEs than for individual ACLAs, anti–β2-GPI, or anti-PT. These findings correspond with those of a recently published study in adults.43 The strong associations result from improved specificity of multiple APLA positivity for the risk of TEs, but sensitivity is decreased because fewer patients have combinations of several APLA subtypes. In the present cohort, LAs as an individual test still showed the strongest association with TEs.
In conclusion, LAs remain the strongest predictor of the risk of TEs in patients with SLE, while other APLA subtypes provide no additional diagnostic value. If testing for LAs is not feasible, ACLA IgM and anti–β2-GPI IgG have the highest predictive value. Lupus anticoagulant and anti–β2-GPI require only single testing, but ACLAs and anti-PT require repeat testing.
Prepublished online as Blood First Edition Paper, September 6, 2005; DOI 10.1182/blood-2005-05-2048.
Supported by a grant from the Canadian Institute of Health Research (grant no. DOP-51711). C.M. is the recipient of an Erwin Schroedinger Scholarship from the Austrian Science Fund. L.M. is a scholar of the Canadian Institutes of Health Research.
C.M. and L.M. designed the research, analyzed data, and wrote the paper; D.F. and H.H. provided vital new reagents and designed the research; P.V. performed the research; and E.S. and M.D. provided study patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr V DeBari, Department of Medicine, Seton Hall University School of Graduate Medical Education, Paterson, NJ, for providing reference calibrators for anti–β2-GPI IgG. We also thank Dr M. Crowther, Dr J. Denburg, and K. Stewart, Department of Medicine, McMaster University, Hamilton, ON, Canada, for providing APLA-positive sera used as standards and controls in anti–β2-GP-I and anti-PT ELISAs.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal