Platelet activation in response to dual stimulation with collagen and thrombin results in the formation of a subpopulation of activated platelets known as coated platelets. Coated platelets are characterized by high surface levels of α-granule proteins and phosphatidylserine, which support the assembly of procoagulant protein complexes. Using murine models, we tested the hypothesis that the collagen receptor-associated molecule FcRγ and the transglutaminase factor XIIIA are required for the formation of coated platelets. Following dual stimulation with the collagen receptor agonist convulxin and thrombin, 68% of platelets from C57BL/6 mice acquired the coated platelet phenotype, defined by high surface levels of fibrinogen and von Willebrand factor and decreased binding of the αIIbβ3 activation-dependent antibody PE-JON/A. In FcRγ-/- mice, only 10% of platelets became “coated” after dual stimulation with convulxin plus thrombin (P < .05 vs C57BL/6 platelets). Decreased coated platelet formation in FcRγ-/- platelets was accompanied by decreased annexin V binding (P < .01) and decreased platelet procoagulant activity (P < .05). Platelets from FXIIIA-/- mice did not differ from control platelets in coated platelet formation or annexin V binding. We conclude that FcRγ, but not factor XIIIA, is essential for formation of highly procoagulant coated platelets following dual stimulation with collagen and thrombin.
Introduction
The process of platelet activation involves several distinct signaling events that induce shape change, granule release, aggregation, and rearrangement of membrane phospholipids.1 Under physiologic conditions, these signaling events can be initiated by multiple agonists acting synchronously or synergistically to generate distinct populations of activated platelets. The heterogeneous nature of activated platelets has been demonstrated in several recent studies in which biochemical, morphologic, or physiologic differences were observed among subpopulations of platelets activated by strong agonists such as collagen or thrombin.2-5
A unique subpopulation of activated platelets, characterized by the surface retention of large amounts of the α-granule proteins fibrinogen, factor V, thrombospondin, and von Willebrand factor (VWF), has been described recently by Dale and colleagues.2,6 In addition to retaining high levels of α-granule proteins on their surface, these activated platelets express surface phosphatidylserine and are able to support the assembly of the prothrombinase complex2 and other procoagulant proteins.3,6,7 Because this subpopulation of activated platelets was initially observed after the dual stimulation of human platelets with collagen and thrombin, they were called COAT platelets (an acronym for collagen- and thrombin-activated platelets).2 It is now known, however, that a similar subpopulation of activated platelets can be generated by other strong agonists or combinations of agonists.6,8 For this reason, the term “coated platelets” has been proposed to refer to the subpopulation of activated platelets that are coated with high levels of procoagulant proteins.9
During the formation of coated platelets, α-granule proteins are covalently modified with serotonin by a transglutaminase. These serotonin-derivatized α-granule proteins are then retained on the surface of the activated platelet through interactions with multiple low-affinity serotonin binding sites.6,10 The transglutaminase responsible for conjugating serotonin to α-granule proteins has not been identified, but factor XIIIA has been suggested as a possible candidate.6 Platelets contain high levels of factor XIIIA11 and generation of coated platelets in response to stimulation with thrombin and convulxin (a specific agonist for the platelet collagen receptor, glycoprotein VI [GPVI]) can be inhibited by an inactivating anti–factor XIII antibody.6 Platelets also contain other transglutaminases, however, and the exact role of factor XIIIA in the formation of coated platelets is unknown.
The physiologic function of coated platelets is not well defined, but the high levels of procoagulant proteins on their surface suggest that they may provide sites for rapid thrombin generation. Initial studies of human platelets stimulated with varying concentrations of collagen and thrombin showed a close correlation between coated platelet formation and prothrombinase activity.2 More recently, the rate and amplitude of thrombin generation were found to correlate directly with coated platelet formation in an in vitro model of coagulation using monocyte tissue factor as the initiating stimulus.3 Very little is known, however, about the formation or function of coated platelets in mice or other experimental animals.
In this study, we demonstrate that coated platelets are formed efficiently when murine platelets are stimulated with thrombin and convulxin. Using mice deficient in FcRγ, we tested the hypothesis that formation of coated platelets requires an intact GPVI/FcRγ signaling pathway. Finally, using factor XIIIA-deficient mice, we tested the hypothesis that the factor XIII transglutaminase is necessary for formation of coated platelets.
Materials and methods
Reagents, antibodies, and mice
Purified bovine prothrombin, factor Va, factor Xa, and α-thrombin were obtained from Hematological Technologies (Essex Junction, VT). S-2238 was obtained from Chromogenix (Milan, Italy). Ionomycin, bovine serum albumin, apyrase, and prostaglandin E1 (PGE1) were obtained from Sigma Chemical (St Louis, MO). Convulxin was obtained from Centerchem (Norwalk, CT). Fluorescein isothiocyanate (FITC)–labeled anti–mouse CD62P (P-selectin), FITC-labeled annexin V, phycoerythrin (PE)–labeled anti–mouse CD49b, and FITC-labeled antifibrinogen antibody were obtained from BD PharMingen (San Diego, CA). FITC- and PE-labeled JON/A antibody was obtained from Emfret Analytics (Würzburg, Germany). Anti-VWF antibody (ab6994) was obtained from AbCam (Cambridge, MA). Corning ClearPro 96-well polypropylene microplates were obtained from Fisher Scientific (Hanover Park, IL). Complete protease inhibitor, EDTA (ethylenediaminetetraacetic acid) free, was obtained from Roche Diagnostics (Mannheim, Germany).
C57BL/6 mice and FcRγ null mice12 on the C57BL/6 genetic background were obtained from the Jackson Laboratory (Bar Harbor, ME). FXIIIA-/- mice13 were crossed with C57BL/6 mice for a single generation and then backcrossed with FXIIIA-/- mice to generate heterozygous (FXIIIA+/-) and homozygous (FXIIIA-/-) littermates for study. Plasma factor XIII activity was measured using the Pefakit Factor XIII (Pentapharm, Basel, Switzerland). All mice were housed in pathogen-free conditions at the University of Iowa Animal Care Facility under National Institutes of Health guidelines and approved animal care protocols. The study protocol was approved by the University of Iowa Animal Care and Use Committee.
Platelet isolation
Whole blood was obtained by cardiac puncture and washed platelets were isolated as described previously.14 Washed platelets were resuspended in modified Tyrode buffer (134 mM NaCl, 2.9 mM KCl, 0.34 mM Na2HPO4, 12 mM NaHCO3, 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 1 mM MgCl2, 5 mM glucose, 0.05% (wt/vol) fatty acid-free bovine serum albumin, pH 7.35), and platelet concentration was evaluated using a HemaVet cell counter (Drew Scientific, Oxford, CT).
Flow cytometry
Washed platelets were suspended in Tyrode buffer containing 2.0 mM CaCl2 at a concentration of 1 × 107/mL. Platelets were either left unstimulated or stimulated with convulxin (250 ng/mL), thrombin (0.5 U/mL), convulxin plus thrombin, or ionomycin (3 μM) plus thrombin for the indicated times at 37°C without stirring. Platelets were then incubated with the appropriate antibody for 2 minutes unless otherwise indicated. Following this incubation period, the platelets were immediately fixed in 1% paraformaldehyde, diluted, and analyzed on a Becton Dickinson FACscan (San Diego, CA) as described previously.14 Platelets were gated by forward scatter and expression of CD49b (α2 integrin). Appropriate compensation was performed for experiments using 2-color flow cytometry. For time-course experiments, 1% paraformaldehyde was added to terminate the reaction at the indicated time point following agonist stimulation. The indicated antibody was added 1 minute prior to fixation. For experiments in which labeled VWF antibody was used, rabbit anti-VWF was labeled using the Zenon One rabbit IgG labeling kit (Molecular Probes, Eugene, OR) per the manufacturer's recommendations prior to addition to the platelet mixture.
Platelet procoagulant activity
Platelet procoagulant activity was measured in a modified prothrombinase assay.14 Reactions were performed in polypropylene 96-well microplates at 37°C. Washed platelets (3 × 106/well) were either left unstimulated or stimulated with convulxin (250 ng/mL), thrombin (0.5 U/mL), or thrombin plus convulxin at 37°C in modified Tyrode buffer containing 2.9 mM CaCl2 and 0.05% (wt/vol) fatty acid-free bovine serum albumin. After 5 minutes, factor Xa (3 nM) and factor Va (6 nM) were added, followed 1 minute later by prothrombin (4 μM). At 3 minutes, a subsample of the reaction mixture was transferred into Tris (tris(hydroxymethyl)aminomethane)–EDTA cuvette buffer (0.05 M Tris-HCl, 120 mM NaCl, 2.0 mM EDTA, 0.05% bovine serum albumin, pH 7.5). Thrombin activity was measured chromogenically using the substrate S-2238 (3 mM) in a Spectramax 190 microplate spectrophotometer (Molecular Devices, Sunnyvale CA).
Cellular transglutaminase activity
Platelet lysates were prepared by adding an equal volume of 2 × cell lysis buffer (2% Triton X-100, 100 mM Tris, pH 7.3, with complete protease inhibitor, EDTA free) to washed platelets resuspended in Tyrode buffer. Transglutaminase activity was determined using a colorimetric microassay (TG-Covtest; Covalab, Lyon, France) per the manufacturer's recommendations. The TG-Covtest measures the transglutaminase-mediated covalent coupling of biotinylated cadaverine to carbobenzoxy (CBZ)–Gln-Gly–coated plates. Platelet lysates were incubated in CBZ-Gln-Gly–coated wells in the presence of calcium, the reducing agent dithiothreitol, and biotinylated cadaverine. In the presence of active transglutaminase biotinylated cadaverine will be covalently linked to the glutaminyl residue of the CBZ-Gln-Gly peptide. Streptavidin-labeled peroxidase is then added, and peroxidase activity is evaluated using a chromogenic substrate. Activity is reported as milliunits per milligram platelet protein lysates using the supplied guinea pig transglutaminase as the reference standard.
Statistical analysis
The unpaired, 2-tailed Student t test was used to compare values between C57BL/6 and FcRγ-/-, between FXIIIA+/- and FXIIIA-/- mice, and in comparisons of cellular transglutaminase activity between FXIIIA and C57BL/6 mice. P less than .05 was used to define statistical significance. Values are reported as mean plus or minus SE.
Results
Formation of murine coated platelets
Platelets isolated from C57BL/6 mice were either left unstimulated or stimulated with convulxin, thrombin, or convulxin plus thrombin. Two subpopulations of activated platelets with different levels of fibrinogen surface retention were observed. The first subpopulation (indicated by region M1 in Figure 1A), observed after stimulation with a single agonist (either convulxin or thrombin), had modestly increased amounts of surface fibrinogen relative to the unstimulated platelet population. The second subpopulation of activated platelets (indicated by region M2 in Figure 1A), which represented a minor fraction of the platelets following stimulation with thrombin and the predominant fraction after dual stimulation with convulxin plus thrombin, was characterized by a higher level of surface-retained fibrinogen. This second group of activated platelets, which corresponds to the human coated platelet described by Dale et al,6 represented approximately 70% of the platelet population after dual stimulation by convulxin plus thrombin.
When the surface retention of another secreted α-granule protein, VWF, was examined, a similar pattern was observed. Intermediate levels of surface VWF retention occurred when platelets were activated by a single agonist, such as thrombin (indicated by region M1 in Figure 1B), and high levels of surface VWF were seen after dual stimulation with convulxin plus thrombin (indicated by region M2 in Figure 1B). These observations demonstrate that murine platelets form a subpopulation of activated platelets with high surface levels of α-granule proteins similar to coated platelets observed in humans.
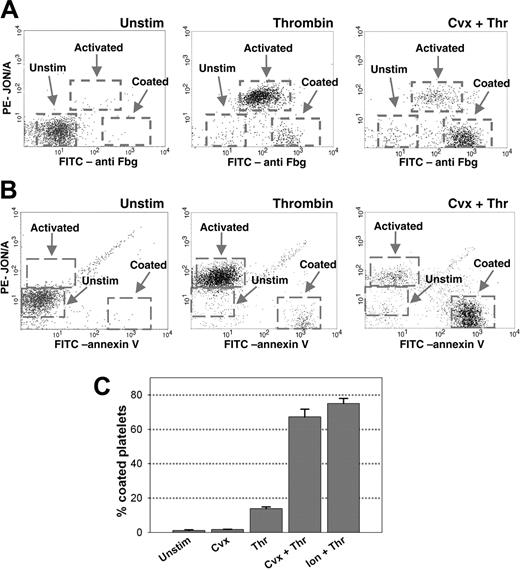
Coated platelets are formed following dual agonist stimulation. C57BL/6 platelets were stimulated with the indicated agonists for 7 minutes and the surface retention of (A) fibrinogen, (B) VWF, or (C) binding of PE-JON/A was measured by flow cytometry. Dashed lines indicate unstimulated platelets; solid lines, agonist-activated platelets. Regions M1 and M2 correspond to populations of activated and coated platelets, respectively. The mean fluorescence for FITC-antifibrinogen (Fbg) on each population in panel A was unstimulated, 9; activated (M1), 80; coated (M2), 718.
Coated platelets are formed following dual agonist stimulation. C57BL/6 platelets were stimulated with the indicated agonists for 7 minutes and the surface retention of (A) fibrinogen, (B) VWF, or (C) binding of PE-JON/A was measured by flow cytometry. Dashed lines indicate unstimulated platelets; solid lines, agonist-activated platelets. Regions M1 and M2 correspond to populations of activated and coated platelets, respectively. The mean fluorescence for FITC-antifibrinogen (Fbg) on each population in panel A was unstimulated, 9; activated (M1), 80; coated (M2), 718.
We next evaluated fibrinogen receptor activation using a recently described monoclonal antibody (PE-JON/A) that specifically recognizes the active form of murine αIIbβ3.15 Seven minutes following activation with the single agonists, thrombin (Figure 1C) or convulxin (data not shown), increased binding of PE-JON/A was observed (indicated by region M1 in Figure 1C) demonstrating the conversion of αIIbβ3 into an active conformation. When convulxin and thrombin were added to platelets simultaneously, however, PE-JON/A binding was increased in only a minority of the activated platelets (Figure 1C). Similar findings were observed using FITC-labeled JON/A (data not shown). The presence of a JON/A-negative population of activated platelets following dual agonist stimulation is reminiscent of the decreased binding of PAC-1, a monoclonal antibody specific for the activated form of αIIbβ3, seen with human coated platelets.6
Further characterization of the murine coated platelet population was performed using 2-color flow cytometry with a FITC-labeled antifibrinogen antibody and PE-JON/A. Following stimulation with convulxin plus thrombin, the majority of platelets exhibited both high levels of platelet surface fibrinogen and low PE-JON/A binding (indicated as “Coated” in Figure 2A). A similar population of coated platelets was seen in only a small percentage of platelets activated by thrombin alone (Figure 2A). The coated platelet population was distinct from the major population of platelets formed following stimulation with thrombin alone (indicated as “Activated” in Figure 2A), which was characterized by moderate levels of surface fibrinogen and increased bound PE-JON/A. Two-color flow cytometry with PE-JON/A and FITC-annexin V, a marker of platelet phosphatidylserine exposure, demonstrated that acquisition of the coated platelet phenotype also correlated with high levels of platelet phosphatidylserine exposure (Figure 2B). Quantification of the coated platelet population demonstrated that 68% ± 4% of C57BL/6 platelets exhibited the coated platelet phenotype after dual stimulation with convulxin plus thrombin, and 74% ± 3% after dual stimulation with ionomycin plus thrombin (Figure 2C).
Further characterization and quantification of coated platelets. Platelets were left unstimulated or activated by the indicated agonists for 7 minutes, stained with (A) FITC-antifibrinogen and PE-JON/A, or (B) FITC-annexin V and PE-JON/A, and analyzed by 2-color flow cytometry. Dashed boxes indicate the unstimulated, activated, and coated platelet populations. (C) Quantification of the coated platelet population following stimulation by various agonists was performed by 2-color flow cytometry with FITC-antifibrinogen and PE-JON/A; n = 5.
Further characterization and quantification of coated platelets. Platelets were left unstimulated or activated by the indicated agonists for 7 minutes, stained with (A) FITC-antifibrinogen and PE-JON/A, or (B) FITC-annexin V and PE-JON/A, and analyzed by 2-color flow cytometry. Dashed boxes indicate the unstimulated, activated, and coated platelet populations. (C) Quantification of the coated platelet population following stimulation by various agonists was performed by 2-color flow cytometry with FITC-antifibrinogen and PE-JON/A; n = 5.
Time course of coated platelet formation
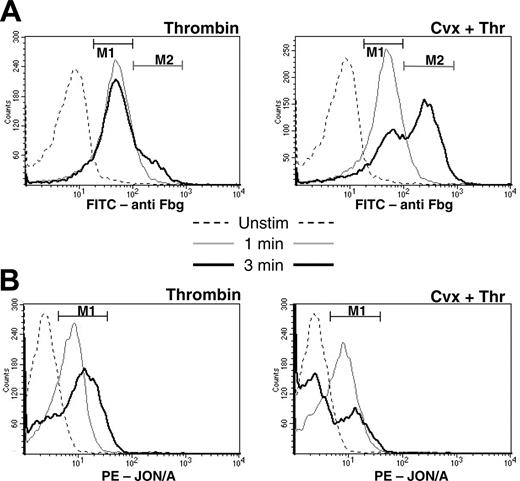
We next examined the time course of coated platelet formation. One minute following stimulation with either the single agonist, thrombin, or the dual agonists, convulxin plus thrombin, only a single population of activated platelets was observed (Figure 3). This population was characterized by an intermediate level of fibrinogen retention (indicated by region M1 in Figure 3A) and increased binding of PE-JON/A (indicated by region M1 in Figure 3B). After 3 minutes, the majority of platelets stimulated with convulxin plus thrombin began to acquire characteristics of the coated platelet with high levels of surface fibrinogen (indicated by region M2 in Figure 3A) and decreased binding of PE-JON/A to a level similar to that of the unstimulated platelet (Figure 3B). Similar time-dependent changes in the pattern of fibrinogen retention and PE-JON/A binding were seen in only a small percentage of platelets stimulated with thrombin alone. These changes in the patterns of antibody recognition following platelet activation suggest that dual agonist stimulation acts as a trigger for subsequent events that result in the maturation of an activated platelet into a coated platelet.
Time course of coated platelet formation. Unstimulated platelets or platelets stimulated with thrombin or thrombin plus convulxin for 1 or 3 minutes were analyzed by flow cytometry with (A) FITC-antifibrinogen or (B) PE-JON/A as described in “Materials and methods.” The absence of agonist is indicated by the dashed line, 1 minute by the thin line, and 3 minutes by the bold line. Regions M1 and M2 correspond to populations of activated and coated platelets, respectively.
Time course of coated platelet formation. Unstimulated platelets or platelets stimulated with thrombin or thrombin plus convulxin for 1 or 3 minutes were analyzed by flow cytometry with (A) FITC-antifibrinogen or (B) PE-JON/A as described in “Materials and methods.” The absence of agonist is indicated by the dashed line, 1 minute by the thin line, and 3 minutes by the bold line. Regions M1 and M2 correspond to populations of activated and coated platelets, respectively.
To better define the relationship between PE-JON/A and bound fibrinogen, PE-JON/A was added either before or 7 minutes after agonist stimulation. After stimulation with thrombin alone, the percentage of platelets with intermediate or high levels of fibrinogen retention in the presence of thrombin was 85% ± 2% when PE-JON/A was added prior to activation and 88% ± 2% when PE-JON/A was added 7 minutes after stimulation. Similarly, minimal effects of PE-JON/A on the percentage of platelets with high levels of fibrinogen retention were observed following dual stimulation with convulxin and thrombin regardless of whether PE-JON/A was added before (54% ± 2%) or after (69% ± 4%) agonist addition. In both thrombin and convulxin/thrombin-stimulated platelets the addition of 2- to 6-fold greater concentrations of PE-JON/A had no additional effects on fibrinogen retention. These results indicate that the timing of PE-JON/A addition in relationship to platelet activation has minimal or no effect on the degree of fibrinogen binding to either activated or coated platelets.
Role of FcRγ in coated platelet formation
FcRγ is required for the surface expression and signaling activity of the major platelet collagen receptor, GPVI. Absence of FcRγ abrogates several collagen-induced platelet activation responses, including α-granule release and fibrinogen receptor activation.1,16 Additionally, a component of the GPVI/FcRγ-dependent pathway is required for platelet procoagulant activity mediated by dual stimulation with collagen plus thrombin.14
To determine if FcRγ-mediated signaling is also required for coated platelet formation, platelet activation responses were compared in platelets isolated from wild-type (C57BL/6) or FcRγ-/- mice. We first examined P-selectin expression, which is a marker of α-granule release (Figure 4A). Following stimulation with convulxin, platelet surface expression of P-selectin was significantly decreased in FcRγ-/- platelets relative to wild-type platelets (P < .05). No difference in P-selectin expression between wild-type and FcRγ-/- platelets was observed following stimulation with thrombin or thrombin plus convulxin. This finding is consistent with the known importance of the GPVI/FcRγ signaling pathway in collagen-mediated α-granule release.1
Formation of coated platelets (defined as activated platelets with high surface fibrinogen and decreased PE-JON/A binding) was not seen with either wild-type or FcRγ-/- platelets after stimulation with convulxin alone (Figure 4B). Stimulation with thrombin alone resulted in a small population of coated platelets (10%-15%) in both wild-type and FcRγ-/- platelets (Figure 4B). On dual stimulation with convulxin plus thrombin, a robust increase in coated platelet formation was seen in wild-type platelets (64%) but not in FcRγ-/- platelets (10%; P < .05; Figure 4B-C). Formation of coated platelets occurred in 60% of FcRγ-/- platelets following activation with ionomycin and thrombin, which indicates that downstream pathways necessary for coated platelet formation are intact in the FcRγ-/- mouse (Figure 4B).
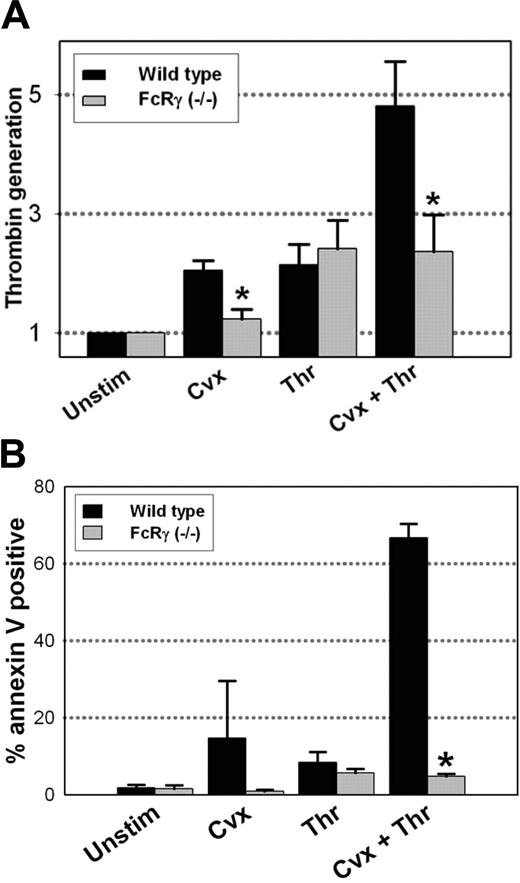
To determine if the defect in coated platelet formation in FcRγ-/- platelets is associated with a decrease in platelet procoagulant activity, platelet-dependent thrombin generation was measured in a prothrombinase assay. Using wild-type platelets, thrombin generation was amplified 5-fold following dual stimulation with convulxin plus thrombin (Figure 5A). Platelet activation with convulxin or thrombin alone resulted in only a slight (1.5- to 2-fold) increase in prothrombinase activity (Figure 5A). When the platelet prothrombinase assay was performed using platelets obtained from FcRγ-/- mice, no significant increase was seen with convulxin alone, and no amplification of platelet prothrombinase activity was observed following dual agonist activation with convulxin plus thrombin.
Coated platelet formation following dual agonist stimulation is greatly decreased in FcRγ-/- platelets. Platelets isolated from wild-type (C57BL/6) (▪) or FcRγ-/- (▦) mice were stimulated with the indicated agonists for 7 minutes. (A) The percentage of platelets with high surface P-selectin expression was measured by flow cytometry with FITC anti-CD62P. (B) Coated platelet formation was evaluated as the percentage of platelets with high fibrinogen, low PE-JON/A binding as indicated in Figure 2B; n = 3. *P < .05 relative to wild-type platelets. (C) FcRγ-/- platelets stained with FITC-antifibrinogen and PE-JON/A and analyzed by 2-color flow cytometry. Dashed boxes indicate the activated and coated platelet populations.
Coated platelet formation following dual agonist stimulation is greatly decreased in FcRγ-/- platelets. Platelets isolated from wild-type (C57BL/6) (▪) or FcRγ-/- (▦) mice were stimulated with the indicated agonists for 7 minutes. (A) The percentage of platelets with high surface P-selectin expression was measured by flow cytometry with FITC anti-CD62P. (B) Coated platelet formation was evaluated as the percentage of platelets with high fibrinogen, low PE-JON/A binding as indicated in Figure 2B; n = 3. *P < .05 relative to wild-type platelets. (C) FcRγ-/- platelets stained with FITC-antifibrinogen and PE-JON/A and analyzed by 2-color flow cytometry. Dashed boxes indicate the activated and coated platelet populations.
The effects of FcRγ deficiency on platelet prothrombinase activity were mirrored by changes in phosphatidylserine exposure as determined by annexin V binding. In wild-type mice, high levels of annexin V binding were seen in a large percentage of platelets (64%) following platelet activation with convulxin plus thrombin (Figure 5B). FcRγ-/- platelets, in comparison, demonstrated minimal phosphatidylserine exposure following dual agonist stimulation (Figure 5B).
Factor XIIIA is not essential for coated platelet formation mice
Factor XIIIA has been proposed to be the transglutaminase responsible for the formation of coated platelets.6 To determine the role of platelet factor XIIIA in coated platelet formation, we measured P-selectin exposure, annexin V binding, and coated platelet formation with platelets isolated from FXIIIA-/- mice. Platelets obtained from FXIIIA-/- mice were compared with those obtained from their heterozygote littermates. Plasma factor XIII activity relative to C57BL/6 mice was less than 1% in FXIIIA-/- mice and 81% ± 4% in FXIIIA+/- mice (P < .01). No differences in P-selectin positivity (Figure 6A) or annexin V binding (Figure 6B) were observed between FXIIIA-/- or FXIIIA+/- mice after stimulation with convulxin or thrombin alone, or following dual stimulation with convulxin plus thrombin. Similarly, when coated platelet formation was examined, no differences could be demonstrated between FXIIIA-/- and FXIIIA+/- platelets (Figure 6C).
Platelet procoagulant activity in FcRγ-/- platelets. (A) Platelets isolated from wild-type (C57BL/6) (▪) or FcRγ-/- (▦) mice were stimulated with the indicated agonists for 5 minutes, and thrombin generation was measured in a prothrombinase assay as described in “Materials and methods”; n = 5. *P < .05 relative to wild-type platelets. (B) Quantification of the platelet population with high levels of annexin V binding in FcRγ-/- and wild-type platelets following stimulation with the indicated agonist for 5 minutes; n = 3. *P < .01 relative to wild-type platelets.
Platelet procoagulant activity in FcRγ-/- platelets. (A) Platelets isolated from wild-type (C57BL/6) (▪) or FcRγ-/- (▦) mice were stimulated with the indicated agonists for 5 minutes, and thrombin generation was measured in a prothrombinase assay as described in “Materials and methods”; n = 5. *P < .05 relative to wild-type platelets. (B) Quantification of the platelet population with high levels of annexin V binding in FcRγ-/- and wild-type platelets following stimulation with the indicated agonist for 5 minutes; n = 3. *P < .01 relative to wild-type platelets.
One plausible explanation for the lack of influence of factor XIII deficiency on coated platelet formation is that other platelet transglutaminases may compensate for the lack of FXIIIA. To examine this possibility, total cellular transglutaminase activity was compared in platelet lysates obtained from control (C57BL/6) mice and FXIIIA-/- mice. Unexpectedly, platelet transglutaminase activity was higher in lysates obtained from FXIIIA-/- mice (0.27 ± 0.03 mU/mg protein) than in the control mice (0.10 ± 0.01 mU/mg protein; P = .03). These findings suggest that there may be a compensatory up-regulation of another transglutaminase in factor XIIIA-deficient platelets.
Discussion
This study demonstrates that coated platelet formation occurs following stimulation of murine platelets with 2 strong agonists, convulxin and thrombin. Characteristic features of the murine coated platelet include high surface retention of α-granule proteins, such as fibrinogen and VWF, decreased binding of a monoclonal antibody specific for the activated form of αIIbβ3, and high level exposure of platelet phosphatidylserine. These features are very similar to those seen in human coated platelets.2,6 In mice, however, coated platelets appear to form more readily than in humans because up to 70% of wild-type murine platelets became coated after stimulation by convulxin and thrombin compared with 30% to 50% of human platelets stimulated with the same agonists.2,3 The other main finding from our study is that FcRγ, but not factor XIIIA, is necessary for formation of murine coated platelets in response to convulxin and thrombin.
A common characteristic of murine and human coated platelets is the decreased recognition of activated αIIbβ3 by a monoclonal antibody specific for the activated form of αIIbβ3. This phenomenon of paradoxically decreased recognition of activated αIIbβ3 on coated platelets has been demonstrated with the PAC-1 antibody in studies with human platelets.6 Because fibrinogen and PAC-1 compete for occupancy of an identical binding site on αIIbβ3, the decreased binding of PAC-1 to αIIbβ3 on the human coated platelet surface has been proposed to be due to an increased affinity of fibrinogen for the activated αIIbβ3 receptor on the surface of the coated platelet relative to PAC-1.6 Unlike PAC-1, however, JON/A recognizes an αIIbβ3 epitope outside of the fibrinogen-binding site and does not compete with fibrinogen for binding of the activated αIIbβ3 receptor.15 In agreement with these findings we found that both FITC- and PE-labeled JON/A bind to “activated” but not “coated” platelets (Figure 2). These results suggest that a different mechanism other than competition for the fibrinogen binding site is responsible for the decreased recognition of αIIbβ3 by JON/A on the coated platelet surface. Possibly, the epitope on αIIbβ3 recognized by JON/A is masked following dual agonist activation with convulxin and thrombin because of a conformational change in αIIbβ3. Future studies that more closely examine the functional conformation of αIIbβ3 on the coated platelet will be required to resolve this question.
FcRγ is a critical component of the GPVI signal transduction pathway, which mediates platelet activation responses to convulxin and collagen.1 Our findings demonstrate that following stimulation of murine platelets with the dual agonists, convulxin plus thrombin, signaling through FcRγ is required for coated platelet formation, platelet phosphatidylserine exposure, and maximal platelet procoagulant activity. The required presence of FcRγ in the initiation of these activities supports a central role for the GPVI/FcRγ signaling pathway in the generation of highly procoagulant coated platelets by the physiologic agonists, collagen and thrombin. These results are in concordance with a model in which tyrosine kinase signaling, triggered by GPVI, is the primary mediator of intracellular signaling by collagen.1 Thrombin, unlike collagen, primarily interacts with receptors known to signal through G protein-regulated pathways, which suggests that interaction of these 2 differentially regulated second messenger pathways may be required for maximal coated platelet formation. Of interest, coated platelet formation occurred normally in FcRγ-/- platelets stimulated with ionomycin plus thrombin, which indicates that ionomycin, a calcium ionophore, can bypass the need for signaling through GPVI/FcRγ.
P-selectin exposure, annexin V binding, and coated platelet formation in FXIIIA-/- platelets. Platelets isolated from FXIIIA+/- (n = 5) (▪) or FXIIIA-/- (n = 7) (▦) mice were stimulated with the indicated agonists for 7 minutes. The percentage of platelets with P-selectin surface expression (A) and annexin V binding (B) was measured by flow cytometry. (C) The percentage of coated platelets, defined as high fibrinogen, low PE-JON/A binding, was determined by 2-color flow cytometry.
P-selectin exposure, annexin V binding, and coated platelet formation in FXIIIA-/- platelets. Platelets isolated from FXIIIA+/- (n = 5) (▪) or FXIIIA-/- (n = 7) (▦) mice were stimulated with the indicated agonists for 7 minutes. The percentage of platelets with P-selectin surface expression (A) and annexin V binding (B) was measured by flow cytometry. (C) The percentage of coated platelets, defined as high fibrinogen, low PE-JON/A binding, was determined by 2-color flow cytometry.
Previous studies using small-molecule transglutaminase inhibitors suggested that a platelet-derived transglutaminase is necessary for coated platelet formation.6 Because monoclonal antibodies to factor XIII were observed to inhibit coated platelet formation, factor XIIIA was proposed as the responsible transglutaminase.6 Up to 50% of the total factor XIII activity in blood is contained within platelets,11 and factor XIIIA has been implicated in the generation of platelet procoagulant activity after adherence to collagen under flow conditions.4 FXIIIA or a closely related transglutaminase has also recently been demonstrated to play an important role in the activation of platelet α-granule secretion,17 and in the enhancement of monocyte activation in atherosclerosis through covalent homodimerization of the angiotensin II receptor.18
Given the proposed important roles of factor XIIIA in these multiple intracellular and extracellular platelet processes, we examined the effect of factor XIIIA deficiency on coated platelet formation in FXIIIA-/- mice. FXIIIA-/- mice had previously been demonstrated to have a bleeding diathesis similar to that seen in factor XIII-deficient humans, including a high incidence of spontaneous hemorrhage and intrauterine pregnancy loss.13,19 Surprisingly, we found that complete absence of factor XIII activity did not affect coated platelet formation, exposure of procoagulant phospholipids, or full expression of P-selectin in murine platelets. These findings indicate that the factor XIII transglutaminase is not necessary for coated platelet formation and suggest that another cellular transglutaminase may compensate for the absence of factor XIIIA. Consistent with this possibility, platelet lysates obtained from FXIIIA-/- mice did not have decreased levels of total transglutaminase activity relative to C57BL/6 mice.
Tissue transglutaminase has previously been detected on both the platelet surface and in platelet lysates,6,20 and factor XIIIA and tissue transglutaminase have similar substrate specificities.21 In the absence of factor XIIIA, platelet levels of tissue transglutaminase may be sufficient to support coated platelet formation. This possibility could perhaps be tested using tissue transglutaminase-deficient mice.22 Another possible reason for the lack of an effect of FXIIIA is that coated platelet formation may occur through a mechanism that does not require transglutaminase-mediated conjugation of serotonin to platelet proteins. Alternatively, species-related differences in the mechanisms of coated platelet formation between humans and mice may explain these results.
In summary, we have characterized the coated platelet phenotype in murine platelets and have demonstrated a close relationship between coated platelet formation and platelet procoagulant activity. The increased thrombin generation on the surface of the coated platelet may be particularly important in facilitating thrombus formation under conditions in which multiple agonists, such as collagen and thrombin, are present. A key advantage of murine models is the ability to examine the mechanistic role of specific gene products in genetically altered mice. Using this approach, we have demonstrated that FcRγ is a key mediator of coated platelet formation in response to dual stimulation with convulxin plus thrombin, but that factor XIIIA is not required for generation of the coated platelet phenotype. Further studies with additional mouse models should provide ever-greater insights into the function and mechanisms of formation of this unique subpopulation of activated platelets.
Prepublished online as Blood First Edition Paper, August 16, 2005; DOI 10.1182/blood-2005-03-1223.
Supported by National Institutes of Health research grants T-32 HL0773 (S.M.J.), HL04460 (J.D.P.), HL63943 (S.R.L.), and CA96691 (T.L.R.) and the National Hemophilia Foundation Clinical Fellowship Program (S.M.J.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal