Numerous p53 target genes have been implicated in DNA damage–induced apoptosis signaling, but proapoptotic Bcl-2 (B-cell leukemia 2) family members of the BH3 (Bcl-2 homolog region [BH] 3)–only subgroup appear to play the critical initiating role. In various types of cultured cells, 3 BH3-only proteins, namely Puma (p53 up-regulated modulator of apoptosis), Noxa, and Bim (Bcl-2 interacting mediator of cell death), have been shown to initiate p53-dependent as well as p53-independent apoptosis in response to DNA damage and treatment with anticancer drugs or glucocorticoids. In particular, the absence of Puma or Bim renders thymocytes and mature lymphocytes refractory to varying degrees to death induced in vitro by growth factor withdrawal, DNA damage, or glucocorticoids. To assess the in vivo relevance of these findings, we subjected mice lacking Puma, Noxa, or Bim to whole-body γ-radiation or the glucocorticoid dexamethasone and compared lymphocyte survival with that in wild-type and BCL2–transgenic mice. Absence of Puma or Bcl-2 overexpression efficiently protected diverse types of lymphocytes from the effects of γ-radiation in vivo, and loss of Bim provided lower but significant protection in most lymphocytes, whereas Noxa deficiency had no impact. Furthermore, both Puma and Bim were found to contribute significantly to glucocorticoid-induced killing. Our results thus establish that Puma and Bim are key initiators of γ-radiation– and glucocorticoid-induced apoptosis in lymphoid cells in vivo.
Introduction
Chemotherapeutic agents and γ-radiation can eliminate malignant as well as normal cells by a variety of mechanisms, but increasing evidence suggests that apoptosis plays a central role, particularly in lymphoid cells.1,2 The apoptosis of lymphocytes in response to genotoxic damage elicited by anticancer drugs or γ-radiation requires p53, the tumor suppressor inactivated in most human malignancies.3 In response to DNA damage or activation of certain oncogenes, wild-type (wt) p53 can either arrest cell cycle progression or induce apoptosis to prevent genome instability and cellular transformation.4 A block to cell-cycle progression in G1 is mediated mainly by p53-regulated transcriptional activation of the cyclin-dependent kinase inhibitor p21(WAF/CIP)5 and G2/M arrest involves other p53 targets, such as the cytoplasmic scaffold protein 14-3-3σ and the proliferating cell nuclear antigen (PCNA)–binding protein termed growth arrest and DNA damage protein 45 (GADD45).6-8 More than 20 genes have been implicated in p53-mediated apoptosis,3 and p53 has also been proposed to have a direct role in cell death initiation at the mitochondrial membrane.9,10
Among the candidate proapoptotic p53 target genes, members of the BH3 (Bcl-2 homology region)–only subgroup of proapoptotic Bcl-2 (B-cell leukemia 2)–like molecules stand out, since these proteins have been recognized as essential initiators of programmed cell death in species as distantly related as Caenorhabditis elegans (C elegans) and mice.11 The BH3-only proteins, such as Bim (Bcl-2 interacting mediator of cell death), Puma (p53 up-regulated modulator of apoptosis), and Noxa, can be activated transcriptionally or after translation in response to developmental cues and cytotoxic stimuli, including anticancer drugs.12 Their proapoptotic activity relies on their ability to bind and antagonize prosurvival members of the Bcl-2 family,13 but the proapoptotic Bcl-2 family members Bax and Bak14 are required for apoptosis induced by BH3-only proteins.15 The Bax (Bcl-2 associated X protein) Bak (Bcl-2 homologous killer)–like proteins are thought to permeabilize the outer mitochondrial membrane, causing release of apoptogenic molecules, such as cytochrome c or Smac/Diablo, that promote activation of the caspases that demolish the cell.15
Resistance to anticancer therapy, particularly in hematologic malignancies, has been associated with overexpression of Bcl-2 or its prosurvival homologs, inactivation of Bax, or loss of p53 function.2 p53 drives the transcription of the BH3-only protein genes Puma16-18 and Noxa,19 and cultured lymphocytes from Puma-deficient mice were shown to be refractory to apoptosis induced by γ-radiation and certain chemotherapeutic drugs.20,21 Noxa appears to play a more restricted role, as it proved dispensable for p53-mediated apoptosis in lymphocytes in vitro but is required for apoptosis in oncogene-transformed mouse embryonic fibroblasts, in which Puma also plays a critical role.21,22 Puma deficiency also protected lymphocytes against certain p53-independent cell death stimuli, such as cytokine deprivation or treatment with glucocorticoids (GCs).20,21 Similarly, Bim is essential for lymphocyte apoptosis induced by cytokine withdrawal or calcium flux, and it was proposed to play a minor role in cell death induced by γ-radiation or treatment with GCs.23 Within the animal, Bim is required for negative selection of autoreactive T cells24-26 as well as B cells27 and for termination of T-cell immune responses,28,29 therefore precluding autoimmunity.
In order to evaluate the physiologic relevance of these in vitro findings for the effects of anticancer therapy in vivo, we examined the responses of mice lacking the BH3-only proteins Puma, Noxa, or Bim to whole-body γ-radiation or the glucocorticoid dexamethasone. The effects on lymphocyte survival were compared with those observed in wild-type mice and animals expressing a vav-BCL2 transgene, which is highly expressed in all lymphoid populations.30 We show that absence of Puma or Bcl-2 overexpression efficiently protects lymphocytes from the effects of DNA damage in vivo and that loss of Bim affords limited protection, mainly in the T-cell linage, whereas absence of Noxa has no protective effect. Moreover, we show that absence of either Bim or Puma provides cell type–specific protection from the effects of GCs.
Materials and methods
Mice
All animal experiments were performed in accordance with the Austrian “Tierversuchsgesetz” and have been granted by the Bundesministerium für Bildung, Wissenschaft und Kultur or were performed according to the guidelines of the Melbourne Directorate Animal Ethics Committee. The generation and genotyping of the Puma-deficient, Noxa-deficient,21 Bim-deficient,23 and vav-BCL2–transgenic mice30 have been described previously. p53-/- mice were kindly donated by Manuel Serrano (Centro Nacional de Investigaciones Oncológicas, Madrid). All mouse strains were on an inbred C57BL/6 genetic background or in the case of the Bim-/- mice had been backcrossed onto this background for at least 12 generations.
Radiation experiments
Adult (7-10 weeks old) mice of the relevant genotypes were exposed to 2.5 or 5.0 Gy γ-radiation in a linear accelerator. Mice were killed by cervical dislocation 20 hours after treatment. Total numbers of cells in bone marrow, thymus, spleen, lymph nodes, and peripheral blood were determined by preparing single-cell suspensions from these tissues and counting an aliquot stained with trypan blue in a hemocytometer.
Glucocorticoid treatment of mice
Adult (7-10 weeks old) mice were injected intraperitoneally with graded doses of the GC dexamethasone, dissolved in normal saline containing 20 μg/mL propyleneglycol. After 20 hours, mice were killed by cervical dislocation.
Immunofluorescence staining, flow cytometric analysis, and cell sorting
Single-cell suspensions from bone marrow, lymph nodes, spleen, and thymus were surface stained with monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC), R-phycoerythrin (PE), allophycocyanin (APC), or biotin (Molecular Probes, Eugene, OR). The monoclonal antibodies used and their specificities are as follows: RA3-6B2, anti-B220; GK1.5, anti-CD4; H129.19.6.8, anti-CD4; 53.6.72, anti-CD8; YTS 169, anti-CD8; RB6-8C5, anti–Gr-1; S7, anti-CD43; 5.1, anti–immunoglobulin M (IgM); 11/26C, anti-IgD; MI/70, anti–Mac-1; Ter119, anti–erythroid cell surface marker; and T24.31.2, anti–Thy-1.
TUNEL staining of tissue sections
To identify apoptotic cells in tissues, sections were freed of paraffin, dehydrated, and incubated for 60 minutes in a humidified incubator with terminal deoxynucleotidyl transferase in the presence of FITC–deoxyuridine triphosphate (dUTP) using an in situ cell death detection kit according to the manufacturer's recommendation (ROCHE, Milan, Italy). Sections were counterstained using 7-aminoactinomycin D (7-AAD; Sigma, St Louis, MO), and incorporated FITC-dUTP terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling–positive (TUNEL+) cells within sections were evaluated using a fluorescence microscope (Zeiss Axiovert 100 M; Carl Zeiss, Heidelberg, Germany) equipped with Plan Neofluar 20 ×/0.5 NA and 40 ×/0.75 NA objective lenses (Zeiss). Sections were embedded in Mowiol/DABCO. Images were acquired using LSM 510, version 2.8 SP1 (Carl Zeiss).
Statistical analysis
Statistical analysis was performed using the Student t test and Stat-view 4.1 software (SAS Institute, Cary, NC). P values of less than .05 were considered to indicate statistically significant differences.
Results
Loss of Puma or Bim protects thymocytes from apoptosis induced by whole-body γ-radiation
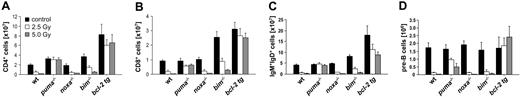
To analyze the requirement of individual BH3-only proteins for DNA damage–induced apoptosis of lymphoid cells in vivo, we exposed wild-type animals and mice lacking Puma, Noxa, or Bim, as well as animals expressing a BCL2 transgene in all hematopoietic cell types,30 to graded doses of γ-radiation. Animals were killed 20 hours thereafter, and thymic glands were harvested and analyzed for their cellularity and cell subset composition as well as for apoptosis in situ. Immature CD4+8+ double-positive (DP) thymocytes are highly susceptible to the effects of γ-radiation,31 and flow cytometric analysis using cell surface marker–specific antibodies demonstrated a dose-dependent decrease in the percentages of these cells in wt and Noxa-deficient animals (Figure 1A,C). In contrast, the percentages of DP thymocytes lacking Puma and Bim or those overexpressing Bcl-2 dropped only marginally (Figure 1A,C). Determination of total thymocyte numbers (Figure 1B) and absolute numbers of CD4+8+ DP cells (Figure 1D) confirmed that Puma-deficient and BCL2–transgenic thymocytes were highly protected from the effects of γ-radiation in vivo and that Bim-/- cells displayed lower, yet significant, resistance, whereas Noxa-/- DP thymocytes were killed as efficiently as wt cells (Figure 1B-D).
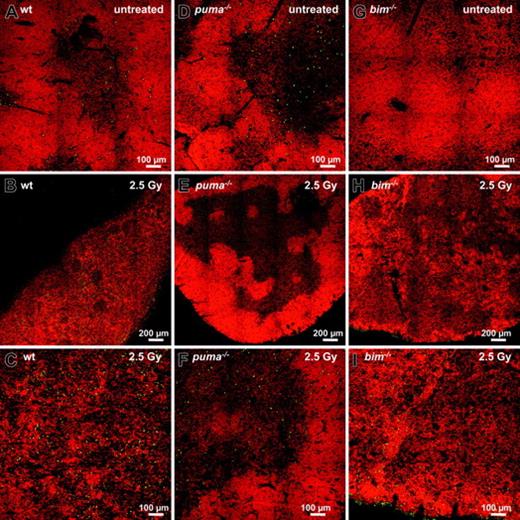
In order to investigate γ-radiation–induced apoptosis in the thymus in situ, we performed TUNEL staining of nicked DNA on thymic sections of wt, Puma-deficient, and Bim-deficient animals. While wt thymic glands had lost their normal architecture within 20 hours after exposure to γ-radiation, those from Puma-deficient mice maintained clearly demarcated cortical and medullary structures (compare Figure 2A-C with 2D-F). Sections from untreated mice of all genotypes showed relatively few TUNEL+ (green) apoptotic cells (Figure 2A,D,G), but numerous green cells became apparent at higher magnification in thymic sections of irradiated wt mice (Figure 2C). In contrast, few green cells appeared in sections from Puma-deficient animals (Figure 2E-F). In sections from Bim-/- mice, although cortical regions were strongly reduced after exposure to γ-radiation (Figure 2H-I), there were fewer TUNEL+ cells than in sections from wt mice (compare Figure 2C with 2I).
Taken together, these experiments demonstrate that in developing T lymphocytes the rate-limiting BH3-only protein for p53-mediated apoptosis in response to γ-radiation in vivo is Puma, but that Bim also plays a minor role, whereas Noxa is dispensable.
Puma and Bim are rate limiting for γ-radiation–induced apoptosis of thymocytes in vivo. Animals of the indicated genotypes were exposed to the indicated doses of whole-body γ-radiation and killed 20 hours thereafter. Thymi were harvested and the single-cell suspensions stained with fluorescence-conjugated antibodies to CD4 and CD8 and analyzed by flow cytometry. (A) Representative dot blots of stained thymocyte suspensions from untreated or radiated animals of each genotype indicating the percentage of CD4-8-, CD4+8+, CD4+8-, and CD4-8+ cells. The lower proportion of CD4+8+ DP cells in the thymus of untreated mice that lack Bim or overexpress Bcl-2 is expected from previous studies.23,30 (B) Thymic cellularity of untreated or radiated animals was assessed using a hemocytometer and trypan blue staining. (C) The percentage of CD4+8+ DP cells and total thymic cellularity were used to calculate (D) the absolute number of CD4+8+ DP thymocytes in control and radiated animals. Bars represent means ± SE of 4 to 10 animals of each genotype and treatment regimen from at least 4 independent experiments. tg indicates transgenic. Statistically significant differences are as follows: (D) 2.5 Gy wt vs Puma-/- (P < .001), 5.0 Gy wt vs Puma-/- (P < .001), 2.5 Gy wt vs Bim-/- (P = .003), 5.0 Gy wt vs Bim-/- (P = .001), 2.5 Gy wt vs vav-BCL2 (P < .001), 5.0 Gy wt vs vav-BCL2 (P < .001).
Puma and Bim are rate limiting for γ-radiation–induced apoptosis of thymocytes in vivo. Animals of the indicated genotypes were exposed to the indicated doses of whole-body γ-radiation and killed 20 hours thereafter. Thymi were harvested and the single-cell suspensions stained with fluorescence-conjugated antibodies to CD4 and CD8 and analyzed by flow cytometry. (A) Representative dot blots of stained thymocyte suspensions from untreated or radiated animals of each genotype indicating the percentage of CD4-8-, CD4+8+, CD4+8-, and CD4-8+ cells. The lower proportion of CD4+8+ DP cells in the thymus of untreated mice that lack Bim or overexpress Bcl-2 is expected from previous studies.23,30 (B) Thymic cellularity of untreated or radiated animals was assessed using a hemocytometer and trypan blue staining. (C) The percentage of CD4+8+ DP cells and total thymic cellularity were used to calculate (D) the absolute number of CD4+8+ DP thymocytes in control and radiated animals. Bars represent means ± SE of 4 to 10 animals of each genotype and treatment regimen from at least 4 independent experiments. tg indicates transgenic. Statistically significant differences are as follows: (D) 2.5 Gy wt vs Puma-/- (P < .001), 5.0 Gy wt vs Puma-/- (P < .001), 2.5 Gy wt vs Bim-/- (P = .003), 5.0 Gy wt vs Bim-/- (P = .001), 2.5 Gy wt vs vav-BCL2 (P < .001), 5.0 Gy wt vs vav-BCL2 (P < .001).
γ-Radiation–induced apoptosis of thymocytes is mediated by a Puma- and Bim-dependent mechanism. Thymic sections derived from wt (A-C), Puma-deficient (D-F), and Bim-deficient (G-I) animals 20 hours after exposure to 2.5 Gy γ-radiation were TUNEL stained using FITC-dUTP to detect nicked DNA in apoptotic cells. Nuclei were counterstained using 7-AAD. Sections were analyzed using a ZEISS Axiovert fluorescence microscope. The top panels show stains of representative thymic sections from untreated animals, revealing comparable numbers of TUNEL+ (green) cells in all genotypes analyzed. After exposure to 2.5 Gy γ-radiation, marginal zone and cortical structures are no longer visible in the wt (B) and are reduced in Bim-/- (H) thymic sections but clearly maintained in the Puma-/- thymus (E). At higher magnification (bottom panels), sections from wt mice (C) exhibit significantly more TUNEL+ apoptotic cells than those from Puma-/- or Bim-/- animals (F,I). The images are representative of 3 or more independent stains performed on organs of at least 2 animals per genotype and treatment.
γ-Radiation–induced apoptosis of thymocytes is mediated by a Puma- and Bim-dependent mechanism. Thymic sections derived from wt (A-C), Puma-deficient (D-F), and Bim-deficient (G-I) animals 20 hours after exposure to 2.5 Gy γ-radiation were TUNEL stained using FITC-dUTP to detect nicked DNA in apoptotic cells. Nuclei were counterstained using 7-AAD. Sections were analyzed using a ZEISS Axiovert fluorescence microscope. The top panels show stains of representative thymic sections from untreated animals, revealing comparable numbers of TUNEL+ (green) cells in all genotypes analyzed. After exposure to 2.5 Gy γ-radiation, marginal zone and cortical structures are no longer visible in the wt (B) and are reduced in Bim-/- (H) thymic sections but clearly maintained in the Puma-/- thymus (E). At higher magnification (bottom panels), sections from wt mice (C) exhibit significantly more TUNEL+ apoptotic cells than those from Puma-/- or Bim-/- animals (F,I). The images are representative of 3 or more independent stains performed on organs of at least 2 animals per genotype and treatment.
Puma mediates γ-radiation–induced apoptosis of mature T and B lymphocytes and B-cell precursors in vivo. Animals of the indicated genotypes were left untreated or exposed to the indicated doses of whole-body γ-radiation and killed 20 hours thereafter. Spleen and bone marrow were harvested, and the single-cell suspensions were counted and stained with fluorescence-conjugated antibodies specific for CD4 and CD8 to identify mature T cells or IgM and IgD to identify mature B cells using a flow cytometer. Bone marrow suspensions were stained using antibodies recognizing B220, IgM, and CD43 in order to identify pre-B-cell precursors. The total cellularity of CD4+ T cells, CD8+ T cells, IgM+D+ B cells in spleen (A-C), and B220+sIgM+CD43- pre-B cells found in the bone marrow of both femora (D) is depicted. Bars represent means ± SE of 4 to 10 animals of each genotype and treatment regimen used in at least 4 independent experiments. Statistically significant differences are as follows: (A) CD4+ T cells: wt versus Puma-/- (P < .001), wt versus Bim-/- (P < .012), wt versus vav-BCL2 (P < .001); (B) CD8+ T cells: wt versus Puma-/- (P < .007), wt versus Bim-/- (P < .013), wt versus vav-BCL2 (P < .002); (C) IgM+D+ B cells: wt versus Puma-/- (P < .001), wt versus Bim-/- (P < .029), wt versus vav-BCL2 (P < .001); (D) B220+sIgM-CD43- pre B cells: wt versus Puma-/- (P < .001), wt versus vav-BCL2 (P < .002).
Puma mediates γ-radiation–induced apoptosis of mature T and B lymphocytes and B-cell precursors in vivo. Animals of the indicated genotypes were left untreated or exposed to the indicated doses of whole-body γ-radiation and killed 20 hours thereafter. Spleen and bone marrow were harvested, and the single-cell suspensions were counted and stained with fluorescence-conjugated antibodies specific for CD4 and CD8 to identify mature T cells or IgM and IgD to identify mature B cells using a flow cytometer. Bone marrow suspensions were stained using antibodies recognizing B220, IgM, and CD43 in order to identify pre-B-cell precursors. The total cellularity of CD4+ T cells, CD8+ T cells, IgM+D+ B cells in spleen (A-C), and B220+sIgM+CD43- pre-B cells found in the bone marrow of both femora (D) is depicted. Bars represent means ± SE of 4 to 10 animals of each genotype and treatment regimen used in at least 4 independent experiments. Statistically significant differences are as follows: (A) CD4+ T cells: wt versus Puma-/- (P < .001), wt versus Bim-/- (P < .012), wt versus vav-BCL2 (P < .001); (B) CD8+ T cells: wt versus Puma-/- (P < .007), wt versus Bim-/- (P < .013), wt versus vav-BCL2 (P < .002); (C) IgM+D+ B cells: wt versus Puma-/- (P < .001), wt versus Bim-/- (P < .029), wt versus vav-BCL2 (P < .001); (D) B220+sIgM-CD43- pre B cells: wt versus Puma-/- (P < .001), wt versus vav-BCL2 (P < .002).
Mature T and B lymphocytes and pre-B cells require Puma for DNA damage–induced apoptosis in vivo
The sensitivity to γ-radiation as well as the molecular requirements for DNA damage–induced apoptosis have been reported to differ between immature thymocytes and mature lymphocytes.1,32 Therefore, we investigated the impact of whole-body γ-radiation on the cellular composition of peripheral lymphoid organs, including spleen, lymph nodes, and bone marrow, as well as peripheral blood. Again, wt, Puma-/-, Noxa-/-, Bim-/-, and vav-BCL2–transgenic mice were killed 20 hours after exposure to graded doses of γ-radiation. Our analysis revealed that loss of Puma or Bcl-2 overexpression conferred significant protection against γ-radiation on all the lymphoid populations examined: CD4+8- and CD4-8+ peripheral T cells (Figure 3A-B), naive IgM+IgDlo B cells (data not shown), mature IgM+IgD+ B cells from spleen (Figure 3C) and lymph nodes (not shown), lymphocytes from peripheral blood (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article), and immature B220+sIgM-CD43- pre-B cells from bone marrow (Figure 3D). Consistent with our previous observations with thymocytes (Figure 1), absence of Bim also provided some protection to mature T and B cells (Figure 3A-C). For example, 20 hours after 2.5 Gy radiation a mean of 28.08% ± 0.5% of wt CD4+ T cells and 20.5% ± 0.2% of wt CD8+ T cells remained, when compared with untreated controls, whereas 40.14% ± 1.5% of Bim-/- CD4+ and 34.61% ± 0.9% of Bim-/- CD8+ T cells survived this treatment (Figure 3A-B). Bim deficiency did not, however, protect the pre-B cells (Figure 3D) and Noxa deficiency had no protective effect in any of the lymphoid populations (Figure 3).
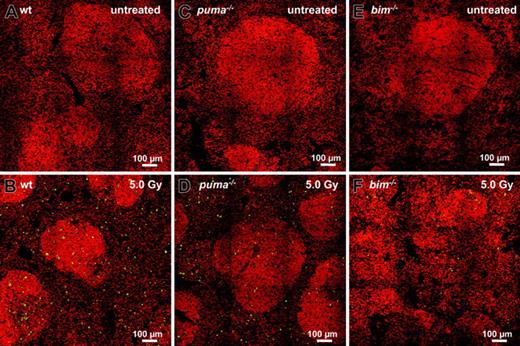
TUNEL staining of untreated spleens failed to reveal apoptotic cells in situ (Figure 4A-C), but the spleens from γ-irradiated wt animals exhibited many apoptotic TUNEL+ green cells (Figure 4B). Consistent with our flow cytometric analysis (Figure 3), sections from irradiated Puma- or Bim-deficient animals reproducibly displayed far fewer TUNEL+ cells than wt sections (Figure 4D-F).
Taken together, these observations indicate that γ-radiation–induced apoptosis of mature T and B cells in vivo depends mainly on Puma but that Bim also contributes significantly. In pre-B cells, γ-radiation–induced death depends primarily on Puma and presumably on other unidentified cell death mediators.
In situ analysis of γ-radiation–induced apoptosis of splenocytes. Sections from untreated (top panels) or γ-radiated (bottom panels) spleens derived from wt (A-B), Puma-deficient (C-D), and Bim-deficient (E-F) animals 20 hours after exposure to 5.0 Gy were TUNEL stained using FITC-dUTP to detect nicked DNA in apoptotic cells. Nuclei were counterstained using 7-AAD. Sections were analyzed using a ZEISS Axiovert fluorescence microscope. Analysis of sections failed to reveal TUNEL+ apoptotic cells in control animals (A,C,E). Numerous TUNEL+ apoptotic cells are visible in sections derived from wt animals (B), but significantly fewer green cells appear in Puma-/- or Bim-/- sections (C,E). Representative images of 3 or more independent stains performed on organs of at least 2 animals per genotype and treatment are shown.
In situ analysis of γ-radiation–induced apoptosis of splenocytes. Sections from untreated (top panels) or γ-radiated (bottom panels) spleens derived from wt (A-B), Puma-deficient (C-D), and Bim-deficient (E-F) animals 20 hours after exposure to 5.0 Gy were TUNEL stained using FITC-dUTP to detect nicked DNA in apoptotic cells. Nuclei were counterstained using 7-AAD. Sections were analyzed using a ZEISS Axiovert fluorescence microscope. Analysis of sections failed to reveal TUNEL+ apoptotic cells in control animals (A,C,E). Numerous TUNEL+ apoptotic cells are visible in sections derived from wt animals (B), but significantly fewer green cells appear in Puma-/- or Bim-/- sections (C,E). Representative images of 3 or more independent stains performed on organs of at least 2 animals per genotype and treatment are shown.
Both Puma and Bim are required for dexamethasone-induced apoptosis of lymphoid cells
GCs are widely used in anticancer therapy and are proving particularly effective in the treatment of certain childhood leukemias.33 Their proapoptotic effects on normal lymphocytes are well documented, but the molecular basis is still poorly understood. Bcl-2 overexpression confers resistance to GCs upon normal thymocytes, pre-B cells, mature T as well as B cells, and lymphoma-derived cell lines,31,34 and GC transcriptional targets include the BH3-only genes Bim and Puma, making them likely candidates for initiating this apoptotic pathway.18,33,35-37 In order to evaluate the in vivo contribution of BH3-only proteins to GC-induced apoptosis of lymphocytes, wt, Puma-/-, Bim-/-, and vav-BCL2–transgenic animals were injected intraperitoneally with graded doses of the therapeutically used glucocorticoid dexamethasone. Animals were killed 20 hours thereafter, and thymic glands, spleens, and bone marrow samples were analyzed for their cellularity and cell subset composition.
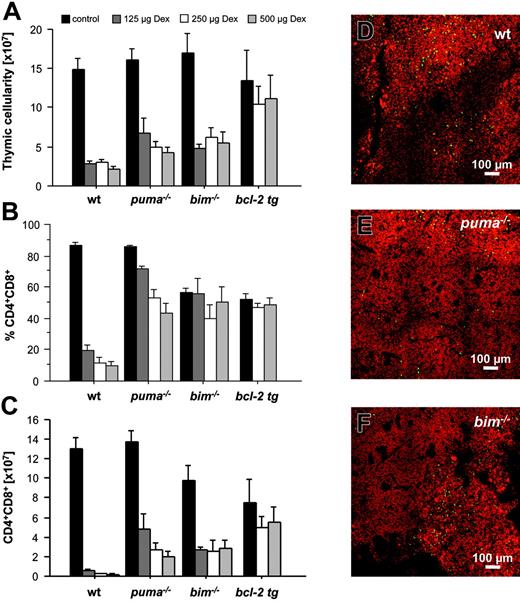
Total thymic cellularity dropped drastically in wt but not in vav-BCL2–transgenic animals (Figure 5A). Total cellularity in Puma- and Bim-deficient thymi was also strongly reduced, but not as pronounced as in thymi from wt animals (Figure 5A). CD4+8+ DP immature thymocytes are particularly sensitive to the effects of GCs, responding with rapid apoptosis.31,34 Immunofluorescent staining and flow cytometric analysis demonstrated a substantial decrease in the percentages of DP thymocytes in dexamethasone-injected wt animals, but not in mice lacking Puma or Bim, or those overexpressing Bcl-2 (Figure 5B). Calculating absolute numbers of CD4+8+ DP cells demonstrated that Puma-deficient as well as Bim-deficient thymocytes were partially protected from the effects of GCs (Figure 5C), and, as shown before,31,34 Bcl-2 overexpression potently protected CD4+8+ DP thymocytes from dexamethasone-induced killing (Figure 5C). Consistent with a minor protective effect of Puma or Bim deficiency, a significant number of TUNEL+ green apoptotic cells was detected, predominantly in cortical regions of thymic sections from wt, Puma-deficient, and Bim-deficient animals (Figure 5D-F and data not shown).
Delay of glucocorticoid-induced apoptosis in Puma- or Bim-deficient thymocytes. Animals of the indicated genotypes were injected intraperitoneally with graded doses of dexamethasone and killed 20 hours later. Thymi were harvested, and single-cell suspensions were counted and stained with fluorescence-conjugated antibodies to CD4 and CD8 and analyzed in a flow cytometer. (A) Total thymic cellularity of saline or dexamethasone-injected animals. (B) The percentage of CD4+8+ double-positive cells and total thymic cellularity were used to calculate (C) the absolute number of immature CD4+8+ DP thymocytes present in control and dexamethasone-treated animals. Bars represent means ± SE of 3 to 6 animals of each genotype and treatment regimen from at least 3 independent experiments. Statistically significant differences are as follows: (C) CD4+8+ DP thymocytes: wt versus Puma-/- (P < .033), wt versus Bim-/- (P < .05), wt versus vav-BCL2 (P < .013). Thymic sections derived from wt (D), Puma-deficient (E), and Bim-deficient (F) animals 20 hours after exposure to 250 μg dexamethasone were TUNEL stained using FITC-dUTP to detect nicked DNA in apoptotic cells. Nuclei were counterstained using 7-AAD. Sections were analyzed using a ZEISS Axiovert fluorescence microscope. The images are representative of 2 independent stains performed on organs of at least 2 animals per genotype and treatment.
Delay of glucocorticoid-induced apoptosis in Puma- or Bim-deficient thymocytes. Animals of the indicated genotypes were injected intraperitoneally with graded doses of dexamethasone and killed 20 hours later. Thymi were harvested, and single-cell suspensions were counted and stained with fluorescence-conjugated antibodies to CD4 and CD8 and analyzed in a flow cytometer. (A) Total thymic cellularity of saline or dexamethasone-injected animals. (B) The percentage of CD4+8+ double-positive cells and total thymic cellularity were used to calculate (C) the absolute number of immature CD4+8+ DP thymocytes present in control and dexamethasone-treated animals. Bars represent means ± SE of 3 to 6 animals of each genotype and treatment regimen from at least 3 independent experiments. Statistically significant differences are as follows: (C) CD4+8+ DP thymocytes: wt versus Puma-/- (P < .033), wt versus Bim-/- (P < .05), wt versus vav-BCL2 (P < .013). Thymic sections derived from wt (D), Puma-deficient (E), and Bim-deficient (F) animals 20 hours after exposure to 250 μg dexamethasone were TUNEL stained using FITC-dUTP to detect nicked DNA in apoptotic cells. Nuclei were counterstained using 7-AAD. Sections were analyzed using a ZEISS Axiovert fluorescence microscope. The images are representative of 2 independent stains performed on organs of at least 2 animals per genotype and treatment.
Cell type–specific contributions of Puma and Bim to glucocorticoid-induced apoptosis of immature and mature lymphocytes in vivo. Animals of the indicated genotypes were injected intraperitoneally with graded doses of dexamethasone and killed 20 hours later. Spleens and bone marrow were harvested, and single-cell suspensions were counted and stained with various fluorescence-conjugated cell surface marker–specific antibodies and analyzed in a flow cytometer. Total numbers of mature CD4+ T cells (A) and CD8+ T cells (B) from spleens of saline or dexamethasone-injected animals. (C) Total cellularity of B220+sIgM-CD43- pre-B cells in both femora of untreated or dexamethasone-treated animals. Bars represent means ± SE of 3 to 6 animals of each genotype and treatment regimen used from at least 3 independent experiments. Statistically significant differences are as follows: (A) CD4+ T cells: wt versus Puma-/- (P < .049); wt versus vav-BCL2 (P < .008); (B) CD8+ T cells: wt versus Puma-/- (P < .035), wt versus vav-BCL2 (P < .007); (C) B220+IgM-CD43- pre B cells: wt versus Bim-/- (P < .022).
Cell type–specific contributions of Puma and Bim to glucocorticoid-induced apoptosis of immature and mature lymphocytes in vivo. Animals of the indicated genotypes were injected intraperitoneally with graded doses of dexamethasone and killed 20 hours later. Spleens and bone marrow were harvested, and single-cell suspensions were counted and stained with various fluorescence-conjugated cell surface marker–specific antibodies and analyzed in a flow cytometer. Total numbers of mature CD4+ T cells (A) and CD8+ T cells (B) from spleens of saline or dexamethasone-injected animals. (C) Total cellularity of B220+sIgM-CD43- pre-B cells in both femora of untreated or dexamethasone-treated animals. Bars represent means ± SE of 3 to 6 animals of each genotype and treatment regimen used from at least 3 independent experiments. Statistically significant differences are as follows: (A) CD4+ T cells: wt versus Puma-/- (P < .049); wt versus vav-BCL2 (P < .008); (B) CD8+ T cells: wt versus Puma-/- (P < .035), wt versus vav-BCL2 (P < .007); (C) B220+IgM-CD43- pre B cells: wt versus Bim-/- (P < .022).
Since mature lymphocytes are sensitive to GCs, albeit much less so than DP thymocytes, we also evaluated the impact of dexamethasone on the survival of mature splenic lymphocytes. While both Puma and Bim contributed to thymocyte apoptosis, selectivity became apparent in the periphery. Puma deficiency reduced the deletion of both CD4+ and CD8+ mature T cells from spleen in response to dexamethasone, but absence of Bim provided no statistically significant degree of protection (Figure 6A-B). Mature wt B-cell numbers also fell about 50% in response to systemic GC treatment, but neither absence of Puma or Bim nor Bcl-2 overexpression prevented this drop (data not shown). In marked contrast to the T cells, absence of Bim potently protected immature pre-B cells from the bone marrow, whereas absence of Puma had no protective effect (Figure 6C). Collectively, our data indicate that both Puma and Bim play critical and, depending on the cell type, partially overlapping roles in GC-induced apoptosis of lymphocytes in vivo.
Discussion
The resistance of malignant cells to γ-radiation or anticancer drugs, a widespread problem during anticancer therapy, is often attributable to elevated levels of prosurvival Bcl-2 family members, inactivation of proapoptotic Bcl-2 family members, or loss of the tumor suppressor p53.1,2 In addition, killing of normal lymphoid and myeloid cells can be a serious unwanted side effect of anticancer therapy. We have used a panel of knock-out and transgenic animals to investigate the in vivo role of the 3 BH3-only proteins, Puma, Noxa, and Bim, in lymphocyte apoptosis induced by γ-radiation and GCs. Quantitative analysis by flow cytometry (Figures 1 and 3) and in situ analysis of the thymus (Figure 2) and spleen (Figure 4) revealed that in the presence of functional p53, Puma is the rate-limiting proapoptotic molecule for γ-radiation–induced apoptosis in diverse lymphoid cells, including thymocytes, mature T and B cells, and pre-B cells (Figures 1, 2, 3, 4). Indeed, the protection conveyed by its loss in thymocytes (Figures 1, 2) and CD4+ and CD8+ mature T cells and B cells (Figure 3) approached that given by the highly expressed vav-BCL2 transgene.
Somewhat surprisingly, both the flow cytometric and the in situ TUNEL analysis also indicated that Bim, which is not a primary target of p53, contributes to γ-radiation–induced death in thymocytes and mature lymphocytes (Figure 1, 2, 3, 4). These data are consistent with previous in vitro observations revealing a small but significant survival advantage for Bim-/- thymocytes23 and granulocytes38 in response to DNA damage caused by γ-radiation or etoposide, respectively. These findings may indicate that genotoxic damage also elicits a p53-independent route to cell death that requires Bim. It is possible that p53-induced genes either influence Bim mRNA expression levels or regulate its protein function after translation by mediating its activation. Alternatively, since both Puma and Bim can bind to the same set of prosurvival Bcl-2 homologs with similar affinities,48 the absence of Bim might simply increase the availability of free prosurvival Bcl-2–like molecules now able to antagonize Puma more effectively. At first sight, the latter model seems unlikely, because it would predict, for example, that loss of Bim would increase the resistance of thymocytes to apoptosis induced by phorbol ester, which requires Puma, and that loss of Puma should render thymocytes resistant to cell death induced by Ca++ flux, which requires Bim, but neither of these is the case (Villunger et al21 and M.E. and A.V., unpublished observations, 2005). However, the visibility of such an indirect beneficial effect may also depend on the pattern of prosurvival homologs expressed in a given cell type.
Bcl-2 overexpression proved more potent than loss of Puma in protecting CD4+8+ DP thymocytes (Figure 1D) and pre-B cells (Figure 3D) from γ-radiation. This might be interpreted that these cells can die in a p53-independent but Bcl-2–blockable manner. That appears unlikely, however, because DNA damage–induced apoptosis independent of p53 has so far been observed only in cycling normal lymphoid or lymphoma cells,32 while most CD4+8+ DP thymocytes and pre-B cells are noncycling and are rendered completely resistant to γ-radiation by loss of p53.32,39,40
At present, we cannot exclude that γ-radiation–induced cell cycle arrest in surviving β-selected CD4-8- double-negative (DN) cells contributes to the net loss of DP thymocytes. However, the limited number of DN cells that dies in response to γ-radiation within 20 hours does so in a strictly p53-dependent, Bcl-2–blockable manner (Figure S2).
Activation of death receptor signaling by γ-radiation–induced activation of p53 also appears unlikely to play a role in these cells, because this death pathway is not inhibited by Bcl-2 overexpression,41,42 and loss of functional FADD or Casp-8, which are essential for all death receptor–mediated killing, does not interfere with DNA-damage–induced apoptosis.41,43,44 The explanation we favor is that p53, directly or indirectly, activates additional BH3-only proteins that can cooperate with Puma in γ-radiation–induced apoptosis of thymocytes and pre-B cells in vivo, although a contribution by other cell death mediators cannot currently be excluded.
The available data from gene knock-out mice, however, do not seem to support a role for several BH3-only candidates: no protection of lymphocytes from the effects of γ-radiation, in vivo or in vitro, has been observed on loss of Noxa, a bona fide transcriptional target of p53 (this study and Shibue et al22 ) nor of Bid,45 Bik,46 or Bad (A.S., unpublished data, 2005). As the most likely candidate, this leaves Bim, which has been suggested to act in parallel to p53 in preventing lymphomagenesis.47 Nevertheless, even BH3-only proteins that on their own do not contribute notably to γ-radiation–induced apoptosis might cooperate with Puma in this process, and generation of mice lacking Puma plus another BH3-only protein (eg, Puma plus Noxa or Puma plus Bim) should clarify this issue. At present, however, the possibility, suggested by other groups,9,10 that p53 has a transcription-independent role in initiating cell death involving its direct action at mitochondria cannot be excluded.
Of interest, Bim-deficient thymocytes of all maturation stages (CD4-8-, CD4+8+, CD4+8-, and CD4-8+) appeared to be equally sensitive to the effects of γ-radiation (Figure 1) or GCs (Figure 5) and died with similar kinetics, whereas in wt animals, as observed before,31 CD4+8+ DP thymocytes are the most sensitive thymocyte subset. This observation explains why the high percentages of CD4+8+ DP cells remaining after radiation (or GC injection) of Bim-/- mice did not lead to a greater increase in the number of surviving CD4+8+ DP cells (compare Figure 1C vs 1D and 5B vs 5C). The molecular basis for this finding is currently under investigation.
Although Noxa mRNA levels were reported to increase in thymocytes after γ-radiation (Oda et al19 ; and L.C. and A.S., unpublished data, 2005), Noxa-deficient lymphocytes of all differentiation stages proved normally sensitive to this death stimulus. This is somewhat surprising, since transcriptional activation of Noxa clearly requires p53.19,22 A plausible explanation arises from the recent finding that Noxa effectively engages only the prosurvival Bcl-2–like molecules Mcl-1 (myeloid cell leukemia 1) and A1 but not Bcl-2 or Bcl-XL.48 Since lymphocyte survival depends mainly on combined action of Bcl-2, Bcl-XL, and Mcl-1,49 the inability of Noxa to engage Bcl-2 and Bcl-XL would explain why its apoptotic role in lymphocytes is far less pronounced than that of Puma and Bim, which engage all the prosurvival family members.48 The generation of compound knock-out mice lacking Puma or Bim as well as Noxa will establish whether Noxa contributes significantly to p53-mediated apoptosis in lymphocytes.
Taken together, our in vivo findings correlate surprisingly well with studies performed on isolated lymphocytes exposed to γ-radiation ex vivo,20-23 indicating that the microenvironment does not play a critical role in the regulation of the DNA-damage response in lymphocytes in vivo. The only indication that the microenvironment may induce different cellular responses can be drawn from our observation that loss of Puma or Bcl-2 overexpression provides only limited protection to peripheral blood lymphocytes (Figure S2). However, this observation could instead mean that damage to the endothelium allows extravasation of lymphocytes into the surrounding tissue and that this endothelial damage is not blocked efficiently by absence of p53 or Puma. This is supported by the fact that peripheral blood lymphocytes also disappeared readily from the blood stream of Puma- and, most importantly, p53-deficient animals, but these same cell types exhibit resistance to γ-radiation–induced death in the spleen (Figure 3, Figure S2, and data not shown).
GCs are an essential component of many protocols for treatment of lymphoid malignancies.33 Childhood T-cell leukemias, for example, are highly sensitive to GCs, and primary sensitivity to GC-induced apoptosis is associated with good prognosis.33 In contrast, primary or treatment-induced resistance to GCs, mostly associated with mutations in the GC receptor (GR), generally indicates an unfavorable course of the disease.36 GC treatment leads to transcriptional activation or repression of numerous target genes, but none was known that induces apoptosis directly until the genes for both Bim and Puma were shown to be targets.18,35,37
Our in vivo analysis of GC sensitivity of lymphoid cells in BH3-only knock-out mice agrees with observations from cultured cells.21,23 Absence of Puma or Bim provided significant protection from GC-induced apoptosis in thymocytes, but overexpressed Bcl-2 was more effective (Figure 5). This indicates that Bim and Puma act in an overlapping manner, and experiments to determine whether Bim/Puma double-deficient thymocytes are completely resistant to GCs have been initiated.
The relative significance of Puma and Bim in GC-induced death seems to vary with cell type. Whereas their role in thymocytes appears comparable (Figure 5A) in mature CD4+ and CD8+ T cells, which are less sensitive to GCs than CD4+8+ DP thymocytes, Puma loss conveyed greater protection than Bim loss and indeed was at least as effective as Bcl-2 overexpression (Figure 6A-B).
In striking contrast, in pre-B cells, loss of Bim provided almost complete resistance to GC treatment, whereas loss of Puma had no protective effect (Figure 6C). These results are in line with observations that Bim is strongly induced by dexamethasone treatment in the GC-sensitive pre-B-cell leukemia cell line 697.35,36 It thus appears that Puma and Bim can be induced and/or activated by GCs in a cell type–specific manner.
Collectively, our experiments demonstrate that Puma is the rate-limiting BH3-only protein mediating p53-induced apoptosis in response to γ-radiation in vivo, whereas Bim plays a minor role and Noxa is dispensable, at least in lymphoid cells. Indeed, except in DP thymocytes, Puma appears responsible for almost all the proapoptotic effects of p53 in the lymphoid compartment. In GC-induced apoptosis of lymphocytes, however, both Puma and Bim have major roles (Figures 5, 6). From the therapeutic perspective, it is noteworthy that both BH3-only proteins can be up-regulated by GCs and other mechanisms that do not require p53.12,18 Hence, exploring their regulation in more detail may well prompt novel strategies for rendering tumors with defective p53 status, or those refractory to glucocorticoid treatment (eg, due to GR mutations), more sensitive to conventional anticancer treatment. Furthermore, it appears likely that the propensity of malignant lymphocytes to induce these genes in response to γ-radiation or GCs will significantly influence treatment outcome. Hence, the ability of tumors to induce Puma and/or Bim expression under different treatment regiments may well provide useful prognostic markers to guide antitumor therapy.
Prepublished online as Blood First Edition Paper, August 23, 2005; DOI 10.1182/blood-2005-04-1595.
Supported by grants from the National Health and Medical Research Council (NHMRC) (Canberra), the Dr Josef Steiner Cancer Research Foundation (Bern), the Leukemia and Lymphoma Society, the National Institutes of Health (NIH) (A.S. and J.M.A.), and the Austrian Science Fund (FWF) Projects R15, START, and SFB021 (“Cell proliferation and cell death in tumors”) (A.V.). V.L. is a DOC-FFORTE Fellow of the Austrian Academy of Science (OEAW).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to F. Müllauer, K. Rossi, and C. Manzl for technical assistance and mouse genotyping; to M. Brennsteiner for animal care; to Prof P. Lukas for enabling radiation experiments; to R. Pfeilschifter for histology sections; and to R. Gruber-Sgonc for help with TUNEL analysis. We thank Profs S. Cory, R. Kofler, G. Wick, S. Kiessling, and all our colleagues in the lab for insightful discussions.
Dr Coultas's current address is Samuel Lunenfeld Research Institute, Rm 884, Mount Sinai Hospital, University of Toronto, 600 University Avenue, Toronto, Ontario M5G 1X5, Canada.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal