Human hematopoietic stem cells (HSCs) are generally regarded as being devoid of the markers expressed by differentiated blood cells, the lineage-specific antigens. However, recent work suggests that genes associated with the myeloid lineage are transcribed in mouse HSCs. Here, we explore whether myeloid genes are actually translated in human HSCs. We show that CD33, CD13, and CD123, well-established myeloid markers, are expressed on human long-term repopulating cells from cord blood and bone marrow. In addition, we demonstrate that nonobese diabetic/severe combined immunodeficiency (NOD/SCID) leukemia-initiating cells (SL-ICs) are restricted to the CD33+ fraction in 11 of 12 acute myeloid leukemia (AML) samples studied, indicating that leukemic stem cells (LSCs) express this antigen. This study changes our view of HSCs and the process of differentiation. Furthermore, based on the phenotypic similarity of HSCs and LSCs, our data provide support for the hypothesis that AML derives from an HSC. Our findings also provide a challenge to contemporary attempts to improve the outcome of AML using myeloid antigen-targeted therapies, given the potential for HSC killing.
Introduction
Hematopoietic stem cells (HSCs) are generally regarded as being devoid of lineage-specific antigens.1-3 As HSCs commit to specific blood cell lineages, lineage markers are expressed. However, it has been noted that genes associated with specific lineages are expressed in cells with a stem cell phenotype.4 This led to the development of the “lineage priming” hypothesis that proposes that HSCs promiscuously express lineage-specific genes prior to commitment.5 Using a conditional knockout mouse, one group recently demonstrated that the myeloid gene lysozyme is transcribed in HSCs.6 This appeared to confirm the “lineage priming” hypothesis.
We decided to take the investigation a step further and asked whether markers previously thought to be restricted to the myeloid lineage are actually expressed by human HSCs. This question is also of considerable clinical importance; a number of therapies that target cells expressing myeloid markers are under development for the treatment of acute myeloid leukemia (AML).7-10 These therapies were designed with the aim of selectively killing leukemic blasts that express myeloid antigens such as CD33,11 while sparing normal HSCs. If HSCs also express myeloid markers, HSCs will be targeted along with leukemic cells. One specific therapy, gemtuzumab ozogamicin (GO), an antibody against CD33 that is conjugated to a cytotoxic agent,7,12,13 is undergoing clinical trials in thousands of patients in the United States and Europe. Prolonged cytopenias have been noted despite successful clearance of leukemic cells in patients receiving GO.12,13 This may reflect HSC killing by GO. Furthermore, although GO may induce remission when given as a sole agent, many patients have relapses.12 This may be because the leukemic stem cells (LSCs) are resistant to the toxin to which the antibody is conjugated.7,14 An alternative explanation is that LSCs, unlike the majority of leukemic blasts, do not express CD33.
In this study, nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice were used to phenotype human HSCs. We show that HSCs express a number of myeloid markers including CD13, CD33, and CD123. We also show that AML LSCs express CD33 and CD13 using similar methodology.15,16 The data overturn the dogma that human HSCs are devoid of myeloid markers and change our view of differentiation. This provides a challenge to contemporary attempts to improve the outcome of AML using myeloid antigen-targeted therapies given the potential for HSC killing. Furthermore, our data indicate that the phenotypes of LSCs and a population of HSCs are similar, consistent with the hypothesis that AML arises from an HSC.17
Materials and methods
Primary cells
Peripheral blood cells were obtained from adult patients with newly diagnosed and relapsed AML at St Bartholomew's Hospital (London, United Kingdom) and the University of Pennsylvania School of Medicine (Philadelphia, PA), after informed consent was provided. We obtained cord blood from mothers attending University College Hospital (London, United Kingdom) and bone marrow from healthy volunteer donors at the University of Pennsylvania, after informed consent was provided. The protocol was approved by the hospital research ethics committees (University of Pennsylvania Review Board and the East London Ethical Committee). Mononuclear cells (MNCs) were obtained by Ficoll-Paque density centrifugation.
Mice
All animal experiments were performed in compliance with Home Office and Cancer Research UK (CRUK) guidelines. NOD/SCID mice and NOD/SCID/β2-microglobulin null (NOD/SCID-β2m-/-) mice were originally obtained from Dr Leonard Schultz (Jackson Laboratory, Bar Harbor, ME) and bred at Charles Rivers Laboratories (Margate, United Kingdom). They were kept in microisolators and fed sterile food and acidified water. Mice aged 8 to 12 weeks were irradiated at 375 rads (137Cs source) up to 24 hours before intravenous injection of cells.
Immunophenotyping
Cells were stained with fluorescein isothiocyanate (FITC)–conjugated anti-CD38 and lineage cocktail 1 (CD3, CD14, CD16, CD19, CD20, CD56), peridinin chlorophyll protein complex (PerCP)–conjugated CD34, phycoerythrin (PE)–conjugated anti-CD33 or anti-CD123, or allophycocyanin (APC)–conjugated CD13 (all antibodies from Becton Dickinson [BD] Biosciences, Oxford, United Kingdom). Analyses were performed on either a BD Life Science Research (LSR) flow cytometer or a BD fluorescence activated cell sorting (FACS) Aria flow cytometer after cells were resuspended in a DAPI (4,6 diamidino-2-phenylindole)–containing solution. Gates were set up to exclude nonviable cells and debris. The negative fraction was determined using appropriate isotype controls.
Analysis of murine bone marrow
Murine marrows were stained with human-specific FITC-conjugated anti-CD19, PE-conjugated anti-CD33, and phycoerythrin-cyanin 5 (PE-Cy5)–conjugated anti-CD45 antibodies, and DAPI. A BD LSR flow cytometer was used for analysis. More than 100 000 events were collected. Engraftment of AML was said to be present if a single population of CD45+CD33+CD19- cells was present. Normal multilineage engraftment was defined by the presence of separate CD45+CD33+ and CD45+CD19+ populations with appropriate scatter characteristics.
Assessment of engraftment potential of AML
Samples were screened to assess whether they had the potential to engraft NOD/SCID and NOD/SCID-β2m-/- mice. MNCs (107) were injected into each mouse. Engraftment of AML was confirmed, where possible, with morphology, phenotyping, and fluorescence in situ hybridization. Samples that showed significantly higher engraftment in NOD/SCID-β2m-/- mice were injected into this strain in sorting experiments.
FACS analysis
We depleted cord blood, bone marrow, and AML samples no. 11 and no. 12 of cells expressing lineage markers using StemSep columns and human progenitor enrichment cocktail (StemCell Technologies, Vancouver, BC, Canada) prior to sorting. The sorting was performed either on a MoFlo cell sorter (DakoCytomation Colorado, Fort Collins, CO) or a BD FACS Aria. Gates were set up to exclude nonviable cells and debris. The purity of sorted fractions was assessed to ensure sort quality. We mixed sorted cells with 500 000 irradiated (1500 rads) accessory cells prior to injection into mice.
Effect of monoclonal antibodies on engraftment
Cells were incubated for 30 minutes at 4°C with either the test antibody or isotype control. The cells were washed prior to injection into irradiated, age- and sex-matched mice. Accessory cells were added after the wash step for cord blood Lin-CD34+CD38- cells. Mice were killed at 6 or 12 weeks and the bone marrow was assessed for engraftment as described (see “Analysis of murine bone marrow”).
Serial transplantation
Mice were humanely killed 6 or 12 weeks after transplantation and bone marrow was obtained. Cells were stained with FITC-conjugated anti–HLA-A, -B, and -C and PE-conjugated anti–mouse CD45 antibodies (BD Biosciences) and DAPI. HLA-A+, -B+, -C+ mouse CD45- cells were collected and injected into irradiated NOD/SCID-β2m-/- mice. Six to 12 weeks later, the bone marrow was assessed for engraftment.
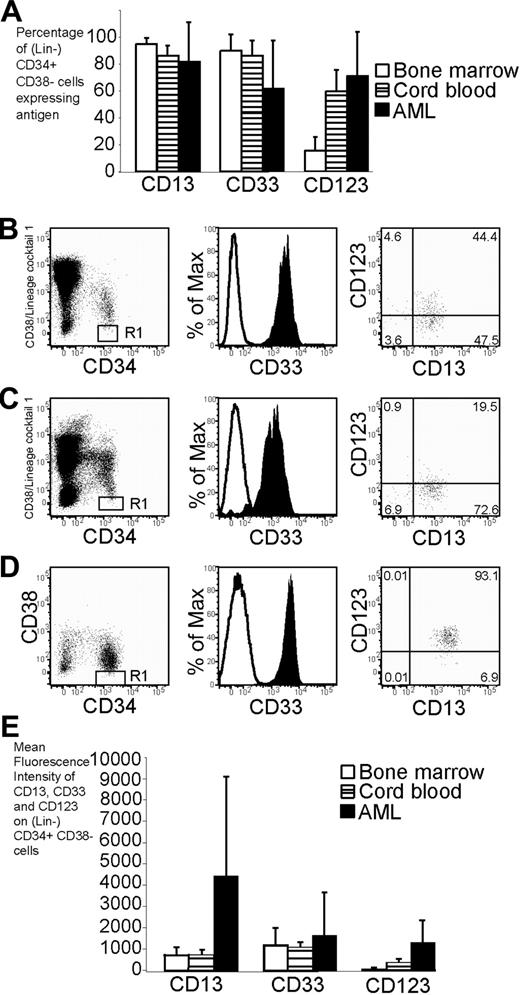
Phenotype of cord blood, bone marrow, and AML cells. Flow cytometry was used to assess the phenotype of cord blood, bone marrow, and AML cells. (A) Percentage of (Lin-) CD34+CD38- cells expressing CD13, CD33, and CD123 in bone marrow (n = 6), cord blood (n = 12), and AML (n = 18). The error bars represent SD. In panels B-D, the expression profile of CD34 and CD38 is shown in the left plot. (Lin-) CD34+CD38- cells are denoted by the R1 gate for further analysis. The expression of CD33 and CD13/CD123 on cells in the R1 gate is shown in the middle and right plots, respectively. Cells stained with isotype control are represented by the open histogram in the middle plot. More than 98% of cells stained with isotype control fall within the bottom left quadrant in the right plot. (B) Expression profile of CD33, CD13, and CD123 on Lin-CD34+CD38- cord blood. (C) Expression profile of CD33, CD13, and CD123 on Lin-CD34+CD38- bone marrow. (D) Expression profile of CD33, CD13, and CD123 on CD34+CD38- AML cells (sample no. 10). (E) Mean fluorescence intensity of antigens on (Lin-) CD34+ CD38- cells from bone marrow, cord blood, and AML.
Phenotype of cord blood, bone marrow, and AML cells. Flow cytometry was used to assess the phenotype of cord blood, bone marrow, and AML cells. (A) Percentage of (Lin-) CD34+CD38- cells expressing CD13, CD33, and CD123 in bone marrow (n = 6), cord blood (n = 12), and AML (n = 18). The error bars represent SD. In panels B-D, the expression profile of CD34 and CD38 is shown in the left plot. (Lin-) CD34+CD38- cells are denoted by the R1 gate for further analysis. The expression of CD33 and CD13/CD123 on cells in the R1 gate is shown in the middle and right plots, respectively. Cells stained with isotype control are represented by the open histogram in the middle plot. More than 98% of cells stained with isotype control fall within the bottom left quadrant in the right plot. (B) Expression profile of CD33, CD13, and CD123 on Lin-CD34+CD38- cord blood. (C) Expression profile of CD33, CD13, and CD123 on Lin-CD34+CD38- bone marrow. (D) Expression profile of CD33, CD13, and CD123 on CD34+CD38- AML cells (sample no. 10). (E) Mean fluorescence intensity of antigens on (Lin-) CD34+ CD38- cells from bone marrow, cord blood, and AML.
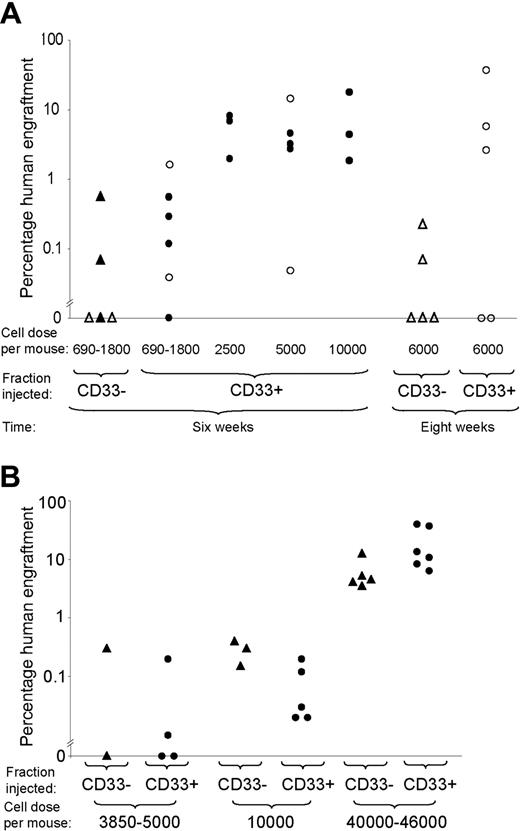
Engraftment of CD33- and CD33+ positive fractions of cord blood and bone marrow. (A) Engraftment of CD33+ and CD33- fractions of cord blood Lin-CD34+CD38- cells in NOD/SCID and NOD/SCID-β2m-/- mice at 6 weeks or 8 weeks. Each symbol represents one mouse. Open symbols represent NOD/SCID mice and filled symbols represent NOD/SCID-β2m-/- mice. Triangles represent mice given injections with CD33- cells and circles represent mice given injections with CD33+ cells. (B) Engraftment of CD33+ and CD33- fractions of bone marrow Lin-CD34+CD38- cells in NOD/SCID-β2m-/- mice at 6 weeks.
Engraftment of CD33- and CD33+ positive fractions of cord blood and bone marrow. (A) Engraftment of CD33+ and CD33- fractions of cord blood Lin-CD34+CD38- cells in NOD/SCID and NOD/SCID-β2m-/- mice at 6 weeks or 8 weeks. Each symbol represents one mouse. Open symbols represent NOD/SCID mice and filled symbols represent NOD/SCID-β2m-/- mice. Triangles represent mice given injections with CD33- cells and circles represent mice given injections with CD33+ cells. (B) Engraftment of CD33+ and CD33- fractions of bone marrow Lin-CD34+CD38- cells in NOD/SCID-β2m-/- mice at 6 weeks.
Statistical methods
L-Calc software (StemCell Technologies) was used to calculate SCID repopulating cell (SRC) frequency. Logistic regression was used to assess significance of difference in SRC frequency and engraftment ability between fractions. Generalized linear models based on the negative binomial distribution were used to assess the statistical significance of the difference between engraftment of samples treated with test antibody and control.
Results
The majority of cord blood Lin-CD34+CD38- cells express myeloid markers
The expression of the myeloid antigens CD13, CD33, and CD123 was examined on the lineage antigen-negative (Lin-) CD34+CD38- population of cord blood and bone marrow. This population contains cells capable of repopulating NOD/SCID bone marrow, termed SCID repopulating cells (SRCs).18 The majority of cord blood and AML (Lin-) CD34+CD38- cells expressed CD13, CD33, and CD123 (Figure 1A-B,D and Figure S1D-I, available on the Blood website; see the Supplemental Figure at the top of the online article). Bone marrow was similar although the percentage of cells expressing CD123 was lower (Figure 1A,C and Figure S1A-C). The intensity of expression of CD123 (but not CD13 or CD33) was significantly higher for AML than either bone marrow or cord blood (P < .05; Figure 1E).
The majority of SRCs express CD33
To investigate whether HSCs express the myeloid marker CD33, cord blood Lin-CD34+CD38- cells were sorted into CD33+ and CD33- fractions. The fractions were injected into NOD/SCID and NOD/SCID-β2m-/- mice and assessed for their ability to repopulate the bone marrow at 6 weeks. Only 2 of the 5 mice that were given transplants of cells from the CD33- fraction showed human multilineage engraftment (Figure 2A). By contrast, 16 of 17 mice given transplants of CD33+ cells showed multilineage engraftment (Figure 2A).
In an additional experiment, NOD/SCID mice were given injections of large doses of CD33- cells (6000 cells/mouse, representing the CD33- fraction of 40 000 Lin-CD34+CD38- cells/mouse) and engraftment was assessed at 8 weeks. Only 2 mice showed multilineage engraftment (Figure 2A). We concluded that the majority of the engrafting potential is present in the CD33+ fraction.
The CD33+ fraction was shown to be capable of self-renewal by performing serial transplantation. Bone marrow cells were harvested from mice that had successfully received transplants of cells from the CD33+ fraction. Human cells (0.9-2 × 106) were injected into 7 secondary recipients. Four of these 7 secondary recipients were engrafted by human cells.
Similar experiments were performed using bone marrow from healthy adults (Figure 2B). The SRC frequency was similar in each fraction, but given that 90% (± 11%) of Lin-CD34+CD38- cells are CD33+ the majority of bone marrow SRCs express CD33+.
Many SRCs express other myeloid markers
We investigated whether cells expressing 2 other myeloid markers, CD13 and CD123, were able to repopulate the bone marrow of immunodeficient mice. Cord blood Lin-CD34+CD38- cells were sorted on the basis of CD13 and CD123 into 4 fractions and injected into NOD/SCID-β2m-/- mice (Table 1). The frequency of SRCs at 6 weeks was significantly higher in the CD13+CD123+ (1 in 575 cells) and CD13-CD123+ (1 in 1157 cells) fractions than in the CD13-CD123- fraction (1 in 4383 cells; P < .05). Indeed, as few as 300 CD13+CD123+ cells could engraft.
Engraftment of CD13+/- CD123+/- fractions of cord blood Lin-CD34+CD38- cells in NOD/SCID-β2m-/- mice and SRC frequency within these fractions
. | No. mice engrafted/no. mice injected . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Cell dose/mouse . | CD13+CD123+* . | CD13+CD123-† . | CD13-CD123+‡ . | CD13-CD123-§ . | |||
| Mice killed at 6 wk | |||||||
| 625 | 3/3 | 1/4 | ND | ND | |||
| 1000-2500 | 6/7 | 4/7 | 5/6 | 4/10 | |||
| More than 6000 | 8/8 | 5/5 | ND | ND | |||
| Mice killed at 12 wk | |||||||
| 1000-1300 | 2/3 | ND | ND | 1/1 | |||
| More than 1700 | ND | 2/2 | 2/2 | 2/2 | |||
. | No. mice engrafted/no. mice injected . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Cell dose/mouse . | CD13+CD123+* . | CD13+CD123-† . | CD13-CD123+‡ . | CD13-CD123-§ . | |||
| Mice killed at 6 wk | |||||||
| 625 | 3/3 | 1/4 | ND | ND | |||
| 1000-2500 | 6/7 | 4/7 | 5/6 | 4/10 | |||
| More than 6000 | 8/8 | 5/5 | ND | ND | |||
| Mice killed at 12 wk | |||||||
| 1000-1300 | 2/3 | ND | ND | 1/1 | |||
| More than 1700 | ND | 2/2 | 2/2 | 2/2 | |||
ND indicates not determined; CI, confidence interval.
SRC frequency: 1 in 575 cells; 95% CI, 1 in 1293 to 1 in 256 cells
SRC frequency: 1 in 1803 cells; 95% CI, 1 in 2604 to 1 in 1249 cells
SRC frequency: 1 in 1157 cells; 95% CI, 1 in 3205 to 1 in 417 cells
SRC frequency: 1 in 4383 cells; 95% CI, 1 in 11 880 to 1 in 1617 cells
Multilineage engraftment was also seen in the more stringent NOD/SCID mouse at 6 weeks from all 4 fractions (Table 2). Together, these data provide further evidence for the presence of myeloid markers on the surface of normal long-term repopulating cells.
Engraftment of CD13+/- CD123+/- fractions of cord blood Lin-CD34+CD38- cells in NOD/SCID mice
. | No. mice engrafted/no. mice injected . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Cell dose/mouse . | CD13+CD123+ . | CD13+CD123- . | CD13-CD123+ . | CD13-CD123- . | |||
| 1500-2000 | ND | ND | 1/2 | 1/2 | |||
| 4500-5000 | 3/6 | 6/6 | ND | ND | |||
| 10000 | 1/1 | 3/3 | ND | ND | |||
. | No. mice engrafted/no. mice injected . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Cell dose/mouse . | CD13+CD123+ . | CD13+CD123- . | CD13-CD123+ . | CD13-CD123- . | |||
| 1500-2000 | ND | ND | 1/2 | 1/2 | |||
| 4500-5000 | 3/6 | 6/6 | ND | ND | |||
| 10000 | 1/1 | 3/3 | ND | ND | |||
ND indicates not determined.
AML LSCs express CD33 and CD13
The expression of myeloid markers on the CD34+CD38- population of AML that is known to contain the SCID-leukemia initiating cells (SL-ICs)16 was studied. The majority of these cells expressed CD13, CD33, and CD123 (Figure 1A,D). MNCs from patients with AML (Table 3) were sorted into CD33+ and CD33- fractions and injected into immunodeficient mice (Figure 3A-D). The purity was 98.8% (± 1.6%) and 97.3% (± 1.9%) for the CD33+ and CD33- fractions, respectively. NOD/SCID or NOD/SCID-β2m-/- mice were used as hosts depending on preliminary screening experiments. At 6 weeks, the bone marrow was assessed for the presence of human AML cells. For 9 of 10 samples engraftment was seen exclusively from the CD33+ fraction (Table 4). Low-level engraftment was seen in one mouse given CD33- cells from one AML (sample no. 7). The dose of cells that was injected from the CD33- fraction matched the engrafting dose from 6 of the 10 samples (Table 4). In addition, Lin-CD34+CD38- cells from 2 samples were sorted into CD33+ and CD33- fractions. Again, engraftment was observed only from the CD33+ fraction. Using logistic regression there is a statistically significant difference in engraftment between the CD33+ and CD33- fractions even when taking into account the dose (P < .05).
Characteristics of AML patient samples
Patient no. . | WBC count, × 109/L . | FAB group . | Karyotype . | CD33, % of MNCs . | CD34+CD38-, % . | CD33, % of CD34+CD38- . |
|---|---|---|---|---|---|---|
| 1 | 151 | 1 | Normal | 76 | 0.24 | 19 |
| 2 | 40 | 2 | Normal | 68 | 0.37 | 26 |
| 3* | 22 | 2 | +11, +13 | 96 | 13.5 | 100 |
| 4* | 66 | 2 | t(8;21) | 98 | 61 | 100 |
| 5 | 73 | 2 | Normal | 94 | 0.68 | 76 |
| 6 | 2.5 | 4 | Normal | 43 | 0.04 | 26 |
| 7 | 71 | 4 | +3, +10 | 88 | 10 | 100 |
| 8 | 115 | 5 | Normal | 87 | 0.04 | 23 |
| 9† | 12 | NA | t(11;19) | 58 | 0.47 | 95 |
| 10† | 19 | NA | Complex | 79 | 31 | 96 |
| 11 | 2.2 | 4 | Normal | 96 | 7.8 | 83 |
| 12 | 77 | 4 | Inv16 | 87 | 10.8 | 96 |
| 13 | 33 | 5 | Normal | 47 | 0.15 | 14 |
Patient no. . | WBC count, × 109/L . | FAB group . | Karyotype . | CD33, % of MNCs . | CD34+CD38-, % . | CD33, % of CD34+CD38- . |
|---|---|---|---|---|---|---|
| 1 | 151 | 1 | Normal | 76 | 0.24 | 19 |
| 2 | 40 | 2 | Normal | 68 | 0.37 | 26 |
| 3* | 22 | 2 | +11, +13 | 96 | 13.5 | 100 |
| 4* | 66 | 2 | t(8;21) | 98 | 61 | 100 |
| 5 | 73 | 2 | Normal | 94 | 0.68 | 76 |
| 6 | 2.5 | 4 | Normal | 43 | 0.04 | 26 |
| 7 | 71 | 4 | +3, +10 | 88 | 10 | 100 |
| 8 | 115 | 5 | Normal | 87 | 0.04 | 23 |
| 9† | 12 | NA | t(11;19) | 58 | 0.47 | 95 |
| 10† | 19 | NA | Complex | 79 | 31 | 96 |
| 11 | 2.2 | 4 | Normal | 96 | 7.8 | 83 |
| 12 | 77 | 4 | Inv16 | 87 | 10.8 | 96 |
| 13 | 33 | 5 | Normal | 47 | 0.15 | 14 |
WBC indicates white blood cell; FAB, French-American-British classification; NA, not applicable.
Samples taken at relapse
Therapy-related AML
Engraftment of CD33+ and CD33- fractions of AML in NOD/SCID mice
Sample no. and dose/mouse . | No. mice with AML/no. mice injected . |
|---|---|
| CD33+ fraction | |
| 1 | |
| 1.0 × 106 | 2/5 |
| 3.0 × 106 | 0/2 |
| 6.0-10.0 × 106 | 6/8 |
| 2 | |
| 1.0 × 106 | 2/3 |
| 6.0 × 106 | 2/2 |
| 3 | |
| 4.0 × 105 | 1/3 |
| 1.0 × 106 | 3/3 |
| 1.0 × 107 | 2/2 |
| 4* | |
| 1.6 × 105 | 2/3 |
| 1.0 × 107 | 2/3 |
| 5* | |
| 5.9 × 105 | 0/2 |
| 8.4 × 106 | 1/1 |
| 6 | |
| 1.5 × 106 | 2/3 |
| 7 | |
| 5.0 × 106 | 0/1 |
| 2.0 × 106 | 0/4 |
| 8 | |
| 2.4 × 106 | 2/3 |
| 9 | |
| 3.8 × 106 | 3/3 |
| 10 | |
| 2.1 × 106 | 0/3 |
| 7.0 × 106 | 1/1 |
| 11*† | |
| 3.5 × 105 | 2/4 |
| 12*† | |
| 2.0 × 106 | 4/4 |
| CD33- fraction | |
| 1 | |
| 1.0 × 106 | 0/5 |
| 3.0 × 106 | 0/3 |
| 2 | |
| 1.0 × 106 | 0/3 |
| 3 | |
| 4.0 × 105 | 0/3 |
| 4* | |
| 1.6 × 105 | 0/3 |
| 5* | |
| 5.9 × 105 | 0/3 |
| 6 | |
| 1.5 × 106 | 0/3 |
| 7 | |
| 7.9 × 105 | 1/3 |
| 2.0 × 106 | 0/5 |
| 8 | |
| 1.0 × 105 | 0/3 |
| 9 | |
| 3.8 × 106 | 0/3 |
| 10 | |
| 2.1 × 106 | 0/3 |
| 11*† | |
| 1.2 × 104 | 0/2 |
| 12*† | |
| 8.0 × 103 | 0/2 |
Sample no. and dose/mouse . | No. mice with AML/no. mice injected . |
|---|---|
| CD33+ fraction | |
| 1 | |
| 1.0 × 106 | 2/5 |
| 3.0 × 106 | 0/2 |
| 6.0-10.0 × 106 | 6/8 |
| 2 | |
| 1.0 × 106 | 2/3 |
| 6.0 × 106 | 2/2 |
| 3 | |
| 4.0 × 105 | 1/3 |
| 1.0 × 106 | 3/3 |
| 1.0 × 107 | 2/2 |
| 4* | |
| 1.6 × 105 | 2/3 |
| 1.0 × 107 | 2/3 |
| 5* | |
| 5.9 × 105 | 0/2 |
| 8.4 × 106 | 1/1 |
| 6 | |
| 1.5 × 106 | 2/3 |
| 7 | |
| 5.0 × 106 | 0/1 |
| 2.0 × 106 | 0/4 |
| 8 | |
| 2.4 × 106 | 2/3 |
| 9 | |
| 3.8 × 106 | 3/3 |
| 10 | |
| 2.1 × 106 | 0/3 |
| 7.0 × 106 | 1/1 |
| 11*† | |
| 3.5 × 105 | 2/4 |
| 12*† | |
| 2.0 × 106 | 4/4 |
| CD33- fraction | |
| 1 | |
| 1.0 × 106 | 0/5 |
| 3.0 × 106 | 0/3 |
| 2 | |
| 1.0 × 106 | 0/3 |
| 3 | |
| 4.0 × 105 | 0/3 |
| 4* | |
| 1.6 × 105 | 0/3 |
| 5* | |
| 5.9 × 105 | 0/3 |
| 6 | |
| 1.5 × 106 | 0/3 |
| 7 | |
| 7.9 × 105 | 1/3 |
| 2.0 × 106 | 0/5 |
| 8 | |
| 1.0 × 105 | 0/3 |
| 9 | |
| 3.8 × 106 | 0/3 |
| 10 | |
| 2.1 × 106 | 0/3 |
| 11*† | |
| 1.2 × 104 | 0/2 |
| 12*† | |
| 8.0 × 103 | 0/2 |
NOD/SCID-β2m-/- mice were used as hosts for these AML samples
Lin-CD34+CD38- cells from these samples were sorted into CD33+ and CD33- fractions
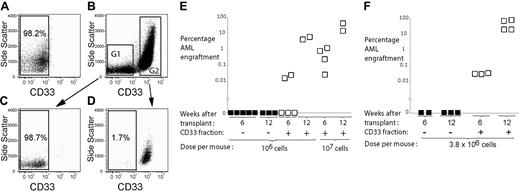
CD33 sorting strategy for AML and engraftment time course of sorted fractions. AML MNCs were stained with anti-CD33 antibody and sorted into CD33- and CD33+ fractions. (A) AML cells stained with isotype control. (B) AML cells stained with anti-CD33 antibody and sort gates G1 and G2. Purity of the sorted CD33- (C) and CD33+ (D) fractions of AML. Engraftment of CD33+ and CD33- fractions of AML samples no. 1 (E) and no. 9 (F) at 6 and 12 weeks. Filled squares represent mice given CD33- cells. Open squares represent mice given CD33+ cells.
CD33 sorting strategy for AML and engraftment time course of sorted fractions. AML MNCs were stained with anti-CD33 antibody and sorted into CD33- and CD33+ fractions. (A) AML cells stained with isotype control. (B) AML cells stained with anti-CD33 antibody and sort gates G1 and G2. Purity of the sorted CD33- (C) and CD33+ (D) fractions of AML. Engraftment of CD33+ and CD33- fractions of AML samples no. 1 (E) and no. 9 (F) at 6 and 12 weeks. Filled squares represent mice given CD33- cells. Open squares represent mice given CD33+ cells.
There is heterogeneity within the SL-IC pool, with some SL-ICs contributing to engraftment only at later time points.19 To ensure that CD33- cells do not contribute to engraftment at a later time point we also assessed engraftment of CD33+ and CD33- fractions at 12 weeks. Three AML samples were studied in this way (samples no. 1, 3, and 9). As with the 6-week studies, AML engraftment was seen exclusively from the CD33+ fraction at 12 weeks. The percentage of AML engraftment at 12 weeks was higher than at 6 weeks (Figure 3E-F).
To explore whether another myeloid marker, CD13, is expressed on SL-ICs, AML samples were sorted into CD13+ and CD13- fractions and injected into mice. Engraftment was seen exclusively from the CD13+ fraction from 2 samples and from both CD13+ and CD13- fractions from an additional 4 samples (Table 5). SL-ICs have been shown to express CD123.20 Therefore, CD13, CD33, and CD123 are expressed on most LSCs.
Engraftment of CD13+ and CD13- fractions of AML in NOD/SCID mice
Sample no. and dose/mouse . | No. mice with AML/no. mice injected . |
|---|---|
| CD13+ fraction | |
| 1* | |
| 5.3 × 105 | 3/4 |
| 3.5 × 106 | 3/3 |
| 3 | |
| 5.0 × 105 | 2/2 |
| 6 | |
| 1.4 × 106 | 2/3 |
| 7 | |
| 5.0 × 106 | 2/2 |
| 3.5 × 106 | 1/2 |
| 9 | |
| 3.6 × 106 | 2/2 |
| 8.3 × 106 | 2/2 |
| 13 | |
| 1.5 × 106 | 3/3 |
| CD13- fraction | |
| 1* | |
| 5.3 × 105 | 0/3 |
| 3 | |
| 5.0 × 105 | 4/4 |
| 3.0 × 106 | 3/3 |
| 6 | |
| 1.4 × 106 | 0/2 |
| 2.7 × 106 | 1/2 |
| 7 | |
| 5.0 × 106 | 1/2 |
| 3.5 × 106 | 4/4 |
| 9 | |
| 3.6 × 106 | 0/3 |
| 13 | |
| 1.5 × 106 | 1/2 |
Sample no. and dose/mouse . | No. mice with AML/no. mice injected . |
|---|---|
| CD13+ fraction | |
| 1* | |
| 5.3 × 105 | 3/4 |
| 3.5 × 106 | 3/3 |
| 3 | |
| 5.0 × 105 | 2/2 |
| 6 | |
| 1.4 × 106 | 2/3 |
| 7 | |
| 5.0 × 106 | 2/2 |
| 3.5 × 106 | 1/2 |
| 9 | |
| 3.6 × 106 | 2/2 |
| 8.3 × 106 | 2/2 |
| 13 | |
| 1.5 × 106 | 3/3 |
| CD13- fraction | |
| 1* | |
| 5.3 × 105 | 0/3 |
| 3 | |
| 5.0 × 105 | 4/4 |
| 3.0 × 106 | 3/3 |
| 6 | |
| 1.4 × 106 | 0/2 |
| 2.7 × 106 | 1/2 |
| 7 | |
| 5.0 × 106 | 1/2 |
| 3.5 × 106 | 4/4 |
| 9 | |
| 3.6 × 106 | 0/3 |
| 13 | |
| 1.5 × 106 | 1/2 |
ND indicates not determined.
Denotes that mice used as a host for this leukemia were NOD/SCID-β2m-/- mice
Anti-CD33 antibody inhibits engraftment of normal and leukemic cells in immunodeficient mice
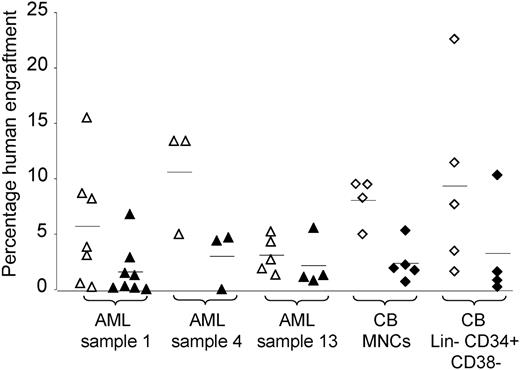
The dose of cells required to engraft mice from the CD33+ fraction was higher than expected from the SL-IC frequency derived from limiting dilution analysis experiments using MNCs (data not shown). To test whether anti-CD33 antibody inhibits engraftment, AML MNCs were incubated with either isotype control or anti-CD33 antibody prior to injection. The engraftment percentage was reduced from cells incubated with anti-CD33 antibody as compared with isotype control in 3 samples (P < .05; Figure 4). The engraftment of one further AML sample (sample no. 7) was abolished by anti-CD33 antibody in one experiment. A similar effect was seen with cord blood MNCs and Lin-CD34+CD38- cells (P < .05; Figure 4). These results provide indirect evidence that SRCs as well as SL-ICs express CD33 and strengthen the data from sorting experiments.
Discussion
In this study, we demonstrate that human cells that express multiple myeloid markers can repopulate the bone marrow of immunodeficient mice and have self-renewal capacity. This indicates that human HSCs are not devoid of myeloid markers but rather express them. Although previous studies have determined that a large proportion of CD34+CD38- cells from a variety of normal hematopoietic tissues express CD33,21-23 no in vivo experiments have addressed the functionality of this population. Here, we demonstrate that normal CD33+ cells are capable of repopulating bone marrow and self-renewal, indicating that the CD33+ fraction contains HSCs. Our in vivo data are in contrast to the in vitro results from one group that found that long-term marrow culture (LTMC)–initiating cells are CD34+CD33-.1-3 This discrepancy may be explained by the different assays used and the inhibitory effect of anti-CD33 antibody reported here as well as in vitro by others.24
Antibodies to CD33 mark a sialic acid-binding immunoglobulin like lectin (Siglec) called Siglec-3.25 Siglecs are thought to deliver an inhibitory signal to the cell on which they reside.26 Binding of anti-CD33 antibody may trigger an inhibitory signal within SL-ICs and SRCs leading to reduced engraftment.
Our study is the first to describe phenotypic heterogeneity of SRCs within the Lin-CD34+CD38- population. This heterogeneity is consistent with the heterogeneity observed within SRCs using viral tracking studies.27 We do not yet know whether all human repopulating cells express at least one myeloid marker. Further studies will be required to delineate the functional properties of repopulating cells based on their expression of CD13, CD33, and CD123.
Our data are consistent with previous studies on transcription of lineage-associated genes within mouse HSCs. It has been reported that mouse HSCs transcribe myeloid but not lymphoid-associated genes.6,28 Given these data and the fact that the lymphoid lineages are thought to have evolved after the myeloid lineages, we speculate that the default position of HSCs is to differentiate along the myeloid lineage.
Our data, showing that most SL-ICs express CD33, suggest that CD33 is a suitable target to allow delivery of therapies to the LSC pool. Treatment failures following administration of CD33-targeted toxin are therefore likely to be due to LSC resistance to the toxin. This is consistent with previous work looking at treatment failures following GO and drug resistance proteins.7,14 However, our finding that CD33 is also on HSCs is worrisome in the context of CD33-targeted therapies, given the potential for killing HSCs. This may explain why prolonged cytopenias sometimes develop despite clearance of leukemic blasts following administration of GO.12,13 However, CD33-based therapies could still be used prior to allogeneic stem cell transplantation to enhance LSC killing.
Effect of anti-CD33 antibody on engraftment of AML and cord blood cells. Anti-CD33 antibody (or isotype control) was incubated with either AML or cord blood cells prior to injection into NOD/SCID (AML samples no. 1 and no. 13 and cord blood MNCs) or NOD/SCID-β2m-/- (sample no. 4 and cord blood Lin-CD34+CD38- cells) mice. Each symbol represents one mouse. Open symbols represent mice given injections of cells incubated with isotype control. Filled symbols represent mice given cells incubated with anti-CD33 antibody. Triangles represent mice given injections with AML and diamonds represent mice given injections with cord blood. Horizontal bars represent mean percentage engraftment.
Effect of anti-CD33 antibody on engraftment of AML and cord blood cells. Anti-CD33 antibody (or isotype control) was incubated with either AML or cord blood cells prior to injection into NOD/SCID (AML samples no. 1 and no. 13 and cord blood MNCs) or NOD/SCID-β2m-/- (sample no. 4 and cord blood Lin-CD34+CD38- cells) mice. Each symbol represents one mouse. Open symbols represent mice given injections of cells incubated with isotype control. Filled symbols represent mice given cells incubated with anti-CD33 antibody. Triangles represent mice given injections with AML and diamonds represent mice given injections with cord blood. Horizontal bars represent mean percentage engraftment.
The cell of origin of AML is debated.17 Some authors think that AML arises from a committed myeloid progenitor that has acquired self-renewal ability, whereas others believe that AML arises from an HSC that is locked into the myeloid lineage. From this study and others, the extended phenotype of most LSCs is CD34+CD38- CD123+CD33+CD13+/-.16,20 The normal hematopoietic cell with the same phenotype as this is found within the HSC pool. By contrast, committed myeloid progenitors express CD38. Presuming that the phenotype of the cell of origin is not altered by transformation (consistent with one recent study29 ), the data support the hypothesis that AML arises from an HSC, rather than a committed myeloid progenitor.
Not all AMLs can engraft immunodeficient mice.16,30 In particular, most leukemias of the M3 subtype are unable to do so. Our findings in AML are thus only applicable to AMLs capable of engraftment. The LSCs of non–engrafting AMLs may have a different phenotype and may derive from a committed myeloid progenitor.
In conclusion, our data indicate that many human HSCs express myeloid markers, overturning the dogma that HSCs are devoid of lineage-associated markers. Our data also have relevance for contemporary attempts to improve outcomes in AML using myeloid antigen-targeted therapies as well as for our understanding of the process of leukemogenesis.
Prepublished online as Blood First Edition Paper, August 30, 2005; DOI 10.1182/blood-2005-03-1072.
Supported by Cancer Research UK and by a grant (HL64856-01) from the National Institutes of Health (D.B.). D.C.T. and D.J.P. contributed equally to this study.
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the patients and donors for providing samples. We also thank the FACS Laboratory and the Biological Resource Unit at Cancer Research UK for their invaluable expertise. We are grateful to Mike Dexter for his comments on this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal