We assessed the outcome of children with Down syndrome (DS) and acute lymphoblastic leukemia (ALL) receiving contemporary risk-based therapy by evaluating clinical and biologic features and outcome of children with ALL, with or without DS, enrolled in Children's Cancer Group (CCG) protocols between 1983 and 1995. Comparison of characteristics of children with ALL with (ALL-DS; n = 179) or without (ALL-NDS; n = 8268) DS showed no differences in initial white blood cell (WBC) count, central nervous system disease, and risk group. Children with ALL-DS did not present with unfavorable translocations and were older than 1 year of age at diagnosis with ALL. Event-free (56% vs 74%; P < .001) and disease-free (55% vs 73%; P < .001) survival at 10 years was significantly lower in the standard-risk ALL-DS population compared with ALL-NDS, but not in high-risk ALL-DS population (event-free survival, 62% vs 59%; P = .9; disease-free survival, 64% vs 59%; P = .9), and these differences persisted regardless of treatment era (early era [1983-1989] vs recent era [1989-1995]). Multivariate analysis revealed that presence of DS demonstrated an independent significant adverse prognostic effect for the standard-risk population, but not for the high-risk patients. These results suggest that intensification of therapy for patients with ALL-DS is needed to maintain outcome comparable with those of ALL-NDS patients.
Introduction
Children with Down syndrome (DS) are at a 10- to 15-fold increased risk of developing acute leukemia,1 including both acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML).2,3 Although numerous mechanisms for leukemogenesis have been proposed in patients with DS,4 none has been clearly established.
Risk-based therapy for childhood ALL has resulted in a marked improvement in survival in the last 3 decades, with 5-year event-free survival for children with ALL approaching 85%.5-9 Past studies indicate a poorer outcome for children with ALL and DS (ALL-DS) when compared with similarly treated children with ALL without DS (ALL-NDS).3,10-15 Factors cited as contributing to a poorer outcome in the ALL-DS population include a lower rate of remission induction and more treatment-related toxicities.
To evaluate the impact of contemporary risk-based therapy in children with ALL-DS, we performed a retrospective cohort analysis of the clinical characteristics and outcome of children with and without DS who were enrolled on Children's Cancer Group (CCG) therapeutic protocols for newly diagnosed ALL between 1983 and 1995.
Patients, materials, and methods
This study included patients enrolled on successive generations of CCG studies for children with newly diagnosed ALL, encompassing 12 separate clinical trials (CCG-104, CCG-105, CCG-106, CCG-107, CCG-123, CCG-139, CCG-1881, CCG-1882, CCG-1883, CCG-1891, CCG-1901, and CCG-1922) conducted between 1983 and 1995. The eligibility criteria, treatment regimens, and results of these trials have been reported and summarized elsewhere.5,16-26 In general, patients were required to be younger than 21 years of age at diagnosis and to have previously untreated ALL, defined by conventional criteria. Informed consent was obtained from each patient or guardian according to institutional policy following approval of the studies by the local institutional review boards.
For the purpose of this analysis, patients were categorized into standard-risk and high-risk groups using National Cancer Institute (NCI) risk categorization.27 Standard-risk was defined as age at diagnosis between 1 and 9 years and initial white blood cell (WBC) count of less than 50 × 109/L (50 000/μL). High-risk was defined as age at diagnosis younger than 1 year or older than 10 years, or initial WBC count greater than 50 × 109/L (50 000/μL). Although these risk criteria were not followed for treatment decisions with earlier therapeutic protocols, these criteria were used for analyses of disease outcome for the entire cohort in this study, regardless of treatment era. Patients were arbitrarily categorized into an early treatment era (1983-1989, which consisted of the CCG-100 series, and included therapeutic protocols CCG-104, -105, -106, -107, and -123) and a recent treatment era (1989-1995, which consisted of the CCG-1800 series, and included therapeutic protocols CCG-1881, -1882, -1883, -1891, -1901, and -1922). Patients were assigned to DS or NDS categories on the basis of characteristic clinical features and the results of peripheral blood or bone marrow cytogenetic studies.
Analysis of disease outcome was examined as overall survival, event-free survival, and disease-free survival. Overall survival was measured from date of initial diagnosis of ALL to date of death from any cause or date of last contact. Event-free survival was defined as the time to first induction failure, relapse at any site (for those who achieved remission), or death, whichever occurred first. Disease-free survival was defined as the time from the end of induction to marrow relapse or death from progressive disease. For those who did not experience events, overall survival, event-free survival, and disease-free survival were the time to last contact. Survival analysis was conducted using the Kaplan-Meier techniques.28 Associated standard errors were calculated by the method of Peto et al.29 Overall survival, event-free survival, and disease-free survival distributions were compared by the log-rank global chi-square test.30 P values are for 2-sided tests.
Results
This analysis includes 8447 children with newly diagnosed ALL enrolled on 12 consecutive therapeutic protocols conducted by CCG between 1983 and 1995. Of these 8447 patients, 179 (2.1%) also had DS. The cohort had been followed for a median of 6.5 years (range, 0-15.1 years).
Presenting clinical and biologic features
The clinical characteristics of the ALL-DS and ALL-NDS patient populations are provided in Table 1. Comparison of clinical and laboratory characteristics at diagnosis showed no significant differences between ALL-DS and ALL-NDS patients in age at diagnosis, initial WBC count, racial or ethnic distribution, the presence of central nervous system disease at diagnosis, hepatomegaly, and assignment to NCI risk groups. No ALL-DS patients less than 1 year of age were identified in this cohort. Children with ALL-DS were more likely than children with ALL-NDS to have a low platelet count (60.7% vs 47.2%; P < .001) and less likely to have a low hemoglobin level (69.8% vs 78.7%; P = .006) at diagnosis. Patients with ALL-DS were less likely to have a mediastinal mass, significant lymphadenopathy, splenomegaly, or T-cell disease when compared with those with ALL-NDS. ALL-DS patients were more likely to have hypodiploidy and hyperdiploidy than were ALL-NDS patients, and less likely to have pseudodiploidy and high hyperdiploidy (P = .04). ALL-DS patients were more likely to have normal chromosomal structure when compared with ALL-NDS patients (45.5% vs 30.3%). Compared with the ALL-NDS cohort (8.4% vs 0%), ALL-DS patients did not present with any translocations associated with adverse outcome (t(9;22), t(4;11)).
Characteristics of the patient population
Variables . | Down syndrome, no. (%) . | No Down syndrome, no. (%) . | P . |
|---|---|---|---|
| Age at diagnosis of ALL | .09 | ||
| Less than 1 y | 0 (0) | 217 (2.6) | |
| 1 y to 9 y | 137 (76.5) | 6222 (75.3) | |
| 10 y or older | 42 (23.5) | 1822 (22.1) | |
| Clinical characteristics | |||
| CNS disease at diagnosis | 4 (2.2) | 240 (2.9) | .8 |
| Initial WBC count above 50 × 109/L | 28 (15.6) | 1813 (21.9) | .1 |
| Initial platelet count above 50 × 109/L | 108 (60.7) | 3901 (47.2) | <.001 |
| Initial Hb below 100 g/L | 125 (69.8) | 6468 (78.7) | .006 |
| Mediastinal mass | 10 (5.6) | 795 (9.6) | .09 |
| Lymph nodes | 72 (40.2) | 4096 (49.6) | .02 |
| Enlarged spleen | 68 (38) | 4499 (54.4) | <.001 |
| Enlarged liver | 92 (51.4) | 4466 (54.1) | .5 |
| National Cancer Institute risk categories | .2 | ||
| Standard risk | 118 (65.9) | 5009 (60.6) | |
| High risk | 61 (34.1) | 3259 (39.4) | |
| Lineage, n = 4931 | .05 | ||
| B-cell | 94 (92.2) | 4082 (84.5) | |
| T-cell | 8 (7.8) | 747 (15.5) | |
| Chromosomal abnormality | .04 | ||
| Normal | 15 (45.5) | 582 (30.3) | |
| Hypodiploid | 4 (12.1) | 111 (5.8) | |
| Pseudodiploid | 7 (18.2) | 534 (27.8) | |
| Hyperdiploid, 47-50 | 5 (15.2) | 203 (10.6) | |
| High hyperdiploid, above 50 | 4 (9.1) | 491 (25.5) | |
| t(9;22) | 0 (0) | 44 (4.3) | .4 |
| t(4;11) | 0 (0) | 42 (4.1) | |
| Early response day 7 marrow | .7 | ||
| M1 (more than 25% blasts) | 36 (46.8) | 1949 (51.5) | |
| M2 (5%-25% blasts) | 20 (26) | 873 (23.1) | |
| M3 (less than 5% blasts) | 21 (27.3) | 964 (25.5) | |
| Induction results, n = 4807 | |||
| Failure, entire cohort | 6 (5.8) | 70 (1.5) | .002 |
| Failure, standard-risk | 2.9 | 0.3 | .008 |
| Failure, high-risk | 3.1 | 1.4 | .9 |
| Events | |||
| No. patients (%) | 70 (39.1) | 2414 (29.2) | .004 |
| Nature of events, n = 4903 | .02 | ||
| No events | 76 (73.1) | 3661 (76.3) | |
| Death in induction | 3 (2.9) | 40 (0.8) | |
| Induction failure | 3 (2.9) | 37 (0.8) | |
| Marrow relapse | 11 (10.6) | 643 (13.4) | |
| CNS relapse | 3 (2.9) | 203 (4.2) | |
| Testicular relapse | 1 (1) | 45 (0.9) | |
| Other | 0 | 29 (0.6) | |
| Second malignancy | 0 | 20 (0.4) | |
| Death after first event | 7 (6.7) | 121 (2.5) | .01 |
| Relapse | |||
| Marrow | 36 (21.8) | 1530 (19.2) | .4 |
| CNS | 8 (4.8) | 623 (7.8) | .2 |
| Testicular | 3 (1.8) | 265 (3.3) | .3 |
| Survival status | <.001 | ||
| Alive | 125 (69.8) | 6661 (80.6) | |
| Dead | 54 (30.2) | 1607 (19.4) |
Variables . | Down syndrome, no. (%) . | No Down syndrome, no. (%) . | P . |
|---|---|---|---|
| Age at diagnosis of ALL | .09 | ||
| Less than 1 y | 0 (0) | 217 (2.6) | |
| 1 y to 9 y | 137 (76.5) | 6222 (75.3) | |
| 10 y or older | 42 (23.5) | 1822 (22.1) | |
| Clinical characteristics | |||
| CNS disease at diagnosis | 4 (2.2) | 240 (2.9) | .8 |
| Initial WBC count above 50 × 109/L | 28 (15.6) | 1813 (21.9) | .1 |
| Initial platelet count above 50 × 109/L | 108 (60.7) | 3901 (47.2) | <.001 |
| Initial Hb below 100 g/L | 125 (69.8) | 6468 (78.7) | .006 |
| Mediastinal mass | 10 (5.6) | 795 (9.6) | .09 |
| Lymph nodes | 72 (40.2) | 4096 (49.6) | .02 |
| Enlarged spleen | 68 (38) | 4499 (54.4) | <.001 |
| Enlarged liver | 92 (51.4) | 4466 (54.1) | .5 |
| National Cancer Institute risk categories | .2 | ||
| Standard risk | 118 (65.9) | 5009 (60.6) | |
| High risk | 61 (34.1) | 3259 (39.4) | |
| Lineage, n = 4931 | .05 | ||
| B-cell | 94 (92.2) | 4082 (84.5) | |
| T-cell | 8 (7.8) | 747 (15.5) | |
| Chromosomal abnormality | .04 | ||
| Normal | 15 (45.5) | 582 (30.3) | |
| Hypodiploid | 4 (12.1) | 111 (5.8) | |
| Pseudodiploid | 7 (18.2) | 534 (27.8) | |
| Hyperdiploid, 47-50 | 5 (15.2) | 203 (10.6) | |
| High hyperdiploid, above 50 | 4 (9.1) | 491 (25.5) | |
| t(9;22) | 0 (0) | 44 (4.3) | .4 |
| t(4;11) | 0 (0) | 42 (4.1) | |
| Early response day 7 marrow | .7 | ||
| M1 (more than 25% blasts) | 36 (46.8) | 1949 (51.5) | |
| M2 (5%-25% blasts) | 20 (26) | 873 (23.1) | |
| M3 (less than 5% blasts) | 21 (27.3) | 964 (25.5) | |
| Induction results, n = 4807 | |||
| Failure, entire cohort | 6 (5.8) | 70 (1.5) | .002 |
| Failure, standard-risk | 2.9 | 0.3 | .008 |
| Failure, high-risk | 3.1 | 1.4 | .9 |
| Events | |||
| No. patients (%) | 70 (39.1) | 2414 (29.2) | .004 |
| Nature of events, n = 4903 | .02 | ||
| No events | 76 (73.1) | 3661 (76.3) | |
| Death in induction | 3 (2.9) | 40 (0.8) | |
| Induction failure | 3 (2.9) | 37 (0.8) | |
| Marrow relapse | 11 (10.6) | 643 (13.4) | |
| CNS relapse | 3 (2.9) | 203 (4.2) | |
| Testicular relapse | 1 (1) | 45 (0.9) | |
| Other | 0 | 29 (0.6) | |
| Second malignancy | 0 | 20 (0.4) | |
| Death after first event | 7 (6.7) | 121 (2.5) | .01 |
| Relapse | |||
| Marrow | 36 (21.8) | 1530 (19.2) | .4 |
| CNS | 8 (4.8) | 623 (7.8) | .2 |
| Testicular | 3 (1.8) | 265 (3.3) | .3 |
| Survival status | <.001 | ||
| Alive | 125 (69.8) | 6661 (80.6) | |
| Dead | 54 (30.2) | 1607 (19.4) |
CNS indicates central nervous system.
Response to induction therapy
Induction failure (defined as an M3 marrow [> 25% blasts] at day 28) was more common in children with ALL-DS (n = 3, 3%) than in children with ALL-NDS (n = 33, 0.7%; P = .03) (Table 1); subset analysis demonstrated that this difference was restricted to the standard-risk ALL group (standard-risk [SR-ALL], 2.9% vs 0.3%; P = .008; high-risk [HR-ALL], 3.1% vs 1.4%; P = .9). Children with ALL-DS (n = 3, 3%) were more likely to die during remission induction than those with ALL-NDS (n = 40, 0.8%; P = .02).
Relapse
ALL-DS patients on SR protocols were more likely to suffer leukemic relapse than ALL-NDS patients (21 vs 14; P = .04); no difference in relapse occurred between ALL-DS and ALL-NDS patients enrolled on HR protocols.
Survival analysis
Results of the overall survival, event-free survival, and disease-free survival were significantly lower in the ALL-DS cohort than in ALL-NDS (overall survival: 68% vs 77% at 10 years, P < .001; event-free survival: 56% vs 68 at 10 years, P < .001; disease-free survival at 10 years: 54% vs 67%, P = .001) (Table 2 and Figure 1A). As can be seen, 4 late events (after 8 years from diagnosis) occurred in the ALL-DS arm: all were late bone marrow relapses. Three of these 4 patients died of progressive disease, while 1 is currently alive and disease free. Stratification according to treatment era of clinical trials identified differences in event-free survival (Table 2). Event-free survival for the ALL-DS cohort enrolled during the early era (1983-1989, CCG-100 series) was significantly lower than for the corresponding ALL-NDS cohort (43% vs 62% at 10 years; P < .001) (Figure 1B). In contrast, the event-free survival was comparable between the ALL-DS and the ALL-NDS patients treated during the recent era (1989-1995, CCG-1800 series: ALL-DS: 72% vs ALL-NDS: 74.3% at 9 years; P = .3) (Figure 1C).
Survival among patients with and without Down syndrome enrolled on Children's Cancer Group acute lymphoblastic leukemia studies from 1983 to 1995
. | Entire cohort . | . | . | Standard risk . | . | . | High risk . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | ALL-DS, % . | ALL-NDS, % . | P . | ALL-DS, % . | ALL-NDS, % . | P . | ALL-DS, % . | ALL-NDS, % . | P . | ||||||
| Entire cohort | |||||||||||||||
| Overall survival, 10 y | 68.13 ± 3.9 | 77.24 ± 0.5 | <.001 | 70.17 ± 4.9 | 84.87 ± 0.6 | <.001 | 63.32 ± 7.1 | 65.49 ± 1 | .7 | ||||||
| Event-free survival, 10 y | 55.48 ± 4.7 | 67.95 ± 6 | .007 | 56.29 ± 6 | 73.48 ± 0.7 | <.001 | 62.06 ± 6.5 | 59.29 ± 0.9 | .9 | ||||||
| Disease-free survival, 10 y | 54.35 ± 5 | 67.23 ± 0.6 | .001 | 54.59 ± 6.5 | 72.48 ± 0.8 | <.001 | 63.51 ± 6.7 | 58.98 ± 1 | .9 | ||||||
| Early era: 100 series | |||||||||||||||
| Event-free survival, 10 y | 43.33 ± 5.8 | 62.23 ± 0.8 | <.001 | 43.36 ± 7.5 | 68.13 ± 1.1 | <.001 | 51.59 ± 9.7 | 53.28 ± 1.4 | .6 | ||||||
| Recent era: 1800 series | |||||||||||||||
| Event-free survival, 5 y | 71.62 ± 4.6 | 73.20 ± 0.8 | .3 | 73.66 ± 5.4 | 81.48 ± 0.8 | .04 | 72.92 ± 7.7 | 66.96 ± 1.1 | .5 | ||||||
. | Entire cohort . | . | . | Standard risk . | . | . | High risk . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | ALL-DS, % . | ALL-NDS, % . | P . | ALL-DS, % . | ALL-NDS, % . | P . | ALL-DS, % . | ALL-NDS, % . | P . | ||||||
| Entire cohort | |||||||||||||||
| Overall survival, 10 y | 68.13 ± 3.9 | 77.24 ± 0.5 | <.001 | 70.17 ± 4.9 | 84.87 ± 0.6 | <.001 | 63.32 ± 7.1 | 65.49 ± 1 | .7 | ||||||
| Event-free survival, 10 y | 55.48 ± 4.7 | 67.95 ± 6 | .007 | 56.29 ± 6 | 73.48 ± 0.7 | <.001 | 62.06 ± 6.5 | 59.29 ± 0.9 | .9 | ||||||
| Disease-free survival, 10 y | 54.35 ± 5 | 67.23 ± 0.6 | .001 | 54.59 ± 6.5 | 72.48 ± 0.8 | <.001 | 63.51 ± 6.7 | 58.98 ± 1 | .9 | ||||||
| Early era: 100 series | |||||||||||||||
| Event-free survival, 10 y | 43.33 ± 5.8 | 62.23 ± 0.8 | <.001 | 43.36 ± 7.5 | 68.13 ± 1.1 | <.001 | 51.59 ± 9.7 | 53.28 ± 1.4 | .6 | ||||||
| Recent era: 1800 series | |||||||||||||||
| Event-free survival, 5 y | 71.62 ± 4.6 | 73.20 ± 0.8 | .3 | 73.66 ± 5.4 | 81.48 ± 0.8 | .04 | 72.92 ± 7.7 | 66.96 ± 1.1 | .5 | ||||||
ALL-DS indicates patients with both acute lymphoblastic leukemia (ALL) and Down syndrome (DS); ALL-NDS denotes patients with ALL but without Down syndrome.
Event-free survival after ALL in patients with or without DS, by treatment era. (A) Event-free survival in a cohort of 8447 children treated for ALL on CCG therapeutic protocols between 1983 and 1995: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (B) Event-free survival in a cohort of 3544 children treated for ALL on CCG therapeutic protocols between 1983 and 1989 (Early Era): a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (C) Event-free survival in a cohort of 4903 children treated for ALL on CCG therapeutic protocols between 1989 and 1995 (Recent Era): a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome.
Event-free survival after ALL in patients with or without DS, by treatment era. (A) Event-free survival in a cohort of 8447 children treated for ALL on CCG therapeutic protocols between 1983 and 1995: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (B) Event-free survival in a cohort of 3544 children treated for ALL on CCG therapeutic protocols between 1983 and 1989 (Early Era): a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (C) Event-free survival in a cohort of 4903 children treated for ALL on CCG therapeutic protocols between 1989 and 1995 (Recent Era): a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome.
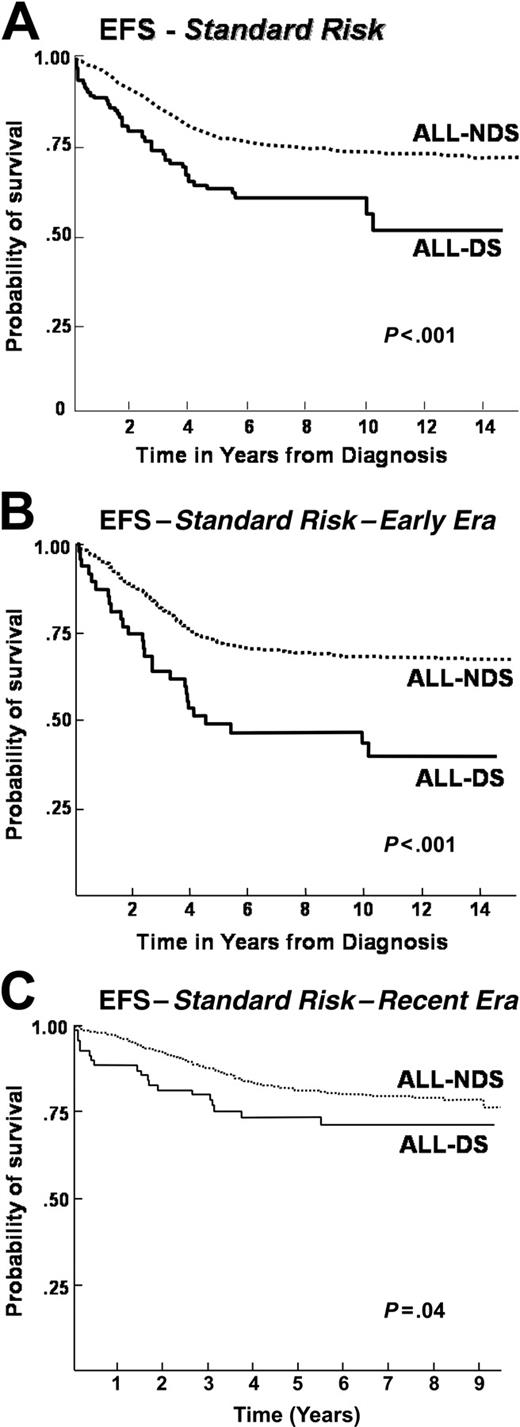
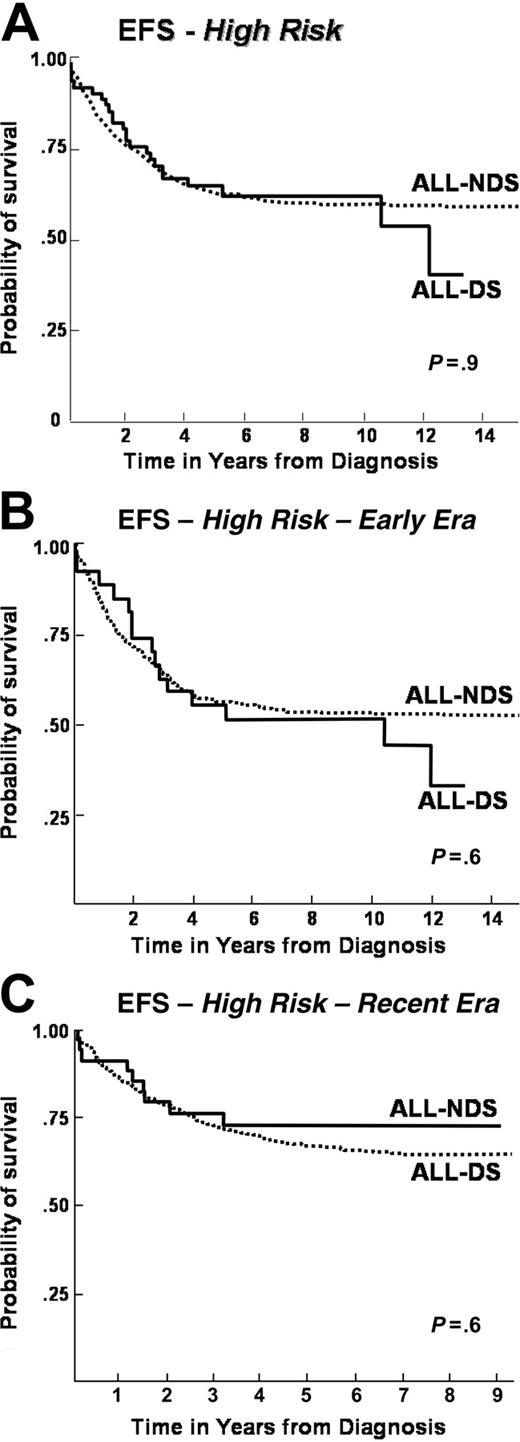
When patients were stratified according to NCI risk groups, differences emerged between ALL-DS and ALL-NDS cohorts. For the standard-risk population, overall, event-free, and disease-free survival rates were significantly lower in the ALL-DS cohort than in the ALL-NDS cohort (overall survival: 70% vs 85% at 10 years, P < .001; event-free survival: 56% vs 74% at 10 years, P < .001; disease-free survival: 55% vs 73% at 10 years, P < .001) (Table 2 and Figure 2A). In contrast, no significant differences in the overall, event-free, and disease-free survival rates were seen between the high-risk ALL-DS and ALL-NDS cohorts (overall survival: 63% vs 66% at 10 years, P = .7; event-free survival: 62% vs 59% at 10 years, P = .9; disease-free survival: 64% vs 59%, P = .9) (Table 2 and Figure 3A).
Further examination of event-free survival by risk groups within each treatment era revealed that the significantly inferior outcome for ALL-DS patients persisted among the standard-risk patients, regardless of treatment era (Figure 2B-C). On the other hand, the event-free survival was comparable for ALL-DS and ALL-NDS patients treated on high-risk protocols, regardless of treatment era (Figure 3B-C).
As seen in Table 3, multivariate analysis of the entire cohort revealed that even after controlling for other significant prognostic factors (WBC and age at diagnosis, race, sex, and liver and spleen size), the presence of DS demonstrated a significant adverse prognostic effect (relative risk [RR] = 1.5; P < .001). Furthermore, in the multivariate analysis, the presence of DS continued to demonstrate a significant adverse prognostic effect in the standard-risk (RR = 2.0; P < .001) but not the high-risk (RR = 1.1; P = .8) population.
Risk factors associated with decreased event-free survival
. | Entire cohort . | . | Standard risk . | . | High risk . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Risk factor . | Relative risk . | P . | Relative risk . | P . | Relative risk . | P . | |||
| Down syndrome | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.5 | <.001 | 2.0 | <.001 | 1.1 | .8 | |||
| Sex | |||||||||
| Male | 1.0 | 1.0 | 1.0 | ||||||
| Female | 0.9 | <.001 | 0.8 | .002 | 0.9 | .009 | |||
| Age | |||||||||
| 1 y to 9 y | 1.0 | 1.0 | |||||||
| Less than 1 y | 3.4 | <.001 | 3.5 | <.001 | |||||
| 10 y or more | 1.7 | <.001 | 1.8 | <.001 | |||||
| White blood cell count at diagnosis, Less than 50 × 109/L | 1.0 | 1.0 | |||||||
| At least 50 × 109/L | 1.4 | <.001 | 1.5 | <.001 | |||||
| Race | |||||||||
| White | 1.0 | 1.0 | 1.0 | ||||||
| Asian | 0.8 | .2 | 1.98 | <.001 | 0.6 | .06 | |||
| Black | 1.4 | <.001 | 1.2 | .1 | 1.1 | .2 | |||
| Hispanic | 1.3 | <.001 | 1.0 | .9 | 1.4 | <.001 | |||
| Spleen size | |||||||||
| Normal | 1.0 | 1.0 | 1.0 | ||||||
| Enlarged | 1.2 | .002 | 1.2 | .006 | 1.1 | .1 | |||
| Liver size | |||||||||
| Normal | 1.0 | 1.0 | 1.0 | ||||||
| Enlarged | 1.2 | <.001 | 1.2 | .007 | 1.2 | .006 | |||
. | Entire cohort . | . | Standard risk . | . | High risk . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Risk factor . | Relative risk . | P . | Relative risk . | P . | Relative risk . | P . | |||
| Down syndrome | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.5 | <.001 | 2.0 | <.001 | 1.1 | .8 | |||
| Sex | |||||||||
| Male | 1.0 | 1.0 | 1.0 | ||||||
| Female | 0.9 | <.001 | 0.8 | .002 | 0.9 | .009 | |||
| Age | |||||||||
| 1 y to 9 y | 1.0 | 1.0 | |||||||
| Less than 1 y | 3.4 | <.001 | 3.5 | <.001 | |||||
| 10 y or more | 1.7 | <.001 | 1.8 | <.001 | |||||
| White blood cell count at diagnosis, Less than 50 × 109/L | 1.0 | 1.0 | |||||||
| At least 50 × 109/L | 1.4 | <.001 | 1.5 | <.001 | |||||
| Race | |||||||||
| White | 1.0 | 1.0 | 1.0 | ||||||
| Asian | 0.8 | .2 | 1.98 | <.001 | 0.6 | .06 | |||
| Black | 1.4 | <.001 | 1.2 | .1 | 1.1 | .2 | |||
| Hispanic | 1.3 | <.001 | 1.0 | .9 | 1.4 | <.001 | |||
| Spleen size | |||||||||
| Normal | 1.0 | 1.0 | 1.0 | ||||||
| Enlarged | 1.2 | .002 | 1.2 | .006 | 1.1 | .1 | |||
| Liver size | |||||||||
| Normal | 1.0 | 1.0 | 1.0 | ||||||
| Enlarged | 1.2 | <.001 | 1.2 | .007 | 1.2 | .006 | |||
Discussion
This cohort of patients with ALL and DS is the largest to date and provides insight into interesting clinical and biological characteristics of ALL in children with DS, as well as their outcome with treatment on contemporary risk-based protocols. We confirmed many previously identified clinical and biologic differences between ALL-DS and ALL-NDS patients. The proportion of patients with Down syndrome in this cohort (2.1%) is similar to that reported by others.3,10,12-15 Again, confirming the findings of other large series, we observed no patients younger than 1 year of age at diagnosis with ALL-DS in this cohort.3,10-14 The absence of ALL-DS in patients less than 1 year of age at diagnosis of ALL is in contrast to a prevalence of 2.6% of patients diagnosed with ALL at an age younger than 1 year in the ALL-NDS cohort. These observations indicate as yet unexplained differences in the biology and pathogenesis of ALL between patients with and without DS.
Event-free survival after standard-risk ALL with or without DS, by treatment era. (A) Event-free survival in a cohort of 5127 children treated for ALL on CCG therapeutic protocols between 1983 and 1995 with standard-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (B) Event-free survival in a cohort of 2118 children treated for ALL on CCG therapeutic protocols between 1983 and 1989 (Early Era) with standard-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (C) Event-free survival in a cohort of 3009 children treated for ALL on CCG therapeutic protocols between 1989 and 1995 (Recent Era) with standard-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome.
Event-free survival after standard-risk ALL with or without DS, by treatment era. (A) Event-free survival in a cohort of 5127 children treated for ALL on CCG therapeutic protocols between 1983 and 1995 with standard-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (B) Event-free survival in a cohort of 2118 children treated for ALL on CCG therapeutic protocols between 1983 and 1989 (Early Era) with standard-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (C) Event-free survival in a cohort of 3009 children treated for ALL on CCG therapeutic protocols between 1989 and 1995 (Recent Era) with standard-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome.
We confirmed other significant differences in clinical features at diagnosis, including a lower platelet count3,11,13 and a lower incidence of splenomegaly,3 lymphadenopathy,3 and mediastinal mass13,14 in ALL-DS than in ALL-NDS patients. Although a study from St Jude Children's Research Hospital reported a lower prevalence of central nervous system involvement in ALL-DS patients,13 our series and others could not confirm this observation.3,11,14,15 Finally, we identified higher hemoglobin levels in ALL-DS patients at diagnosis. This was not seen in an earlier CCG report,3 while Ragab et al12 observed lower hemoglobin levels in patients with ALL-DS than in ALL-NDS.
Our results also confirmed differences observed in the biologic characteristics of leukemic cells between patients with and without DS. Similar to previous reports,10,13-15 leukemic blast with a T-cell immunophenotype was observed less frequently in patients with ALL-DS than in patients with ALL-NDS. Although the Pediatric Oncology Group (POG) experience and an earlier study from St Jude Children's Research Hospital did not show similar results,11,12 their findings may have been limited by sample size, as evidenced by the emergence of a significant difference in the prevalence of T-cell immunophenotype in a subsequent review of a larger St Jude cohort.13 In our study, as in others,10,13-15 the leukemic blasts in ALL-DS patients were less likely to demonstrate chromosomal abnormalities and were less likely to be hyperdiploid than in the ALL-NDS patients. We also observed no adverse chromosomal translocations (t(9;22) or t(4;11)) in patients with ALL-DS, as noted by others.25
In contrast to the superior outcome of children with AML and DS when compared with children with AML without DS,31,32 most reports suggest that the outcome of children with ALL-DS is significantly worse than the outcome of those with ALL-NDS.3,10-13,15 Ragab et al12 noted lower event-free survival in an ALL-DS population that received “conventional” treatment (ie, no consolidation therapy) on POG clinical trials than in the ALL-NDS population, but not in those who received more intensive therapy. Chessells et al15 reported decreased event-free survival for the ALL-DS population, but comparable overall survival to the ALL-NDS population.
The current study demonstrated that children with ALL-DS treated on CCG protocols had decreased overall, event-free, and disease-free survival compared with those with ALL-NDS, and these observations persisted after multivariate analysis to control for other prognostic factors known to affect outcome in children with ALL. Subset analysis based on risk stratification revealed differences between the SR-ALL and HR-ALL cohorts: children with SR-ALL and DS had a worse outcome when compared with children with SR-ALL and NDS; in contrast, children with HR-ALL had similar outcomes regardless of whether they had DS. These differences in outcome by risk category among patients with ALL-DS and ALL-NDS persisted, regardless of the treatment era.
Event-free survival after high-risk ALL with or without DS, by treatment era. (A) Event-free survival in a cohort of 3320 children treated for ALL on CCG therapeutic protocols between 1983 and 1995 with high-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (B) Event-free survival in a cohort of 1426 children treated for ALL on CCG therapeutic protocols between 1983 and 1989 (Early Era) with high-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (C) Event-free survival in a cohort of 1894 children treated for ALL on CCG therapeutic protocols between 1989 and 1995 (Recent Era) with high-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome.
Event-free survival after high-risk ALL with or without DS, by treatment era. (A) Event-free survival in a cohort of 3320 children treated for ALL on CCG therapeutic protocols between 1983 and 1995 with high-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (B) Event-free survival in a cohort of 1426 children treated for ALL on CCG therapeutic protocols between 1983 and 1989 (Early Era) with high-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome. (C) Event-free survival in a cohort of 1894 children treated for ALL on CCG therapeutic protocols between 1989 and 1995 (Recent Era) with high-risk features: a comparison by presence (ALL-DS) or absence (ALL-NDS) of Down syndrome.
We sought to determine the basis for these differences in outcomes by evaluating biologic features of the ALL-DS and ALL-NDS patients in our cohort. Three biologic features with prognostic significance have been identified as being less frequent in ALL-DS patients: (1) hyperdiploidy, (2) adverse translocations, and (3) TEL-AML1 rearrangements.
Hyperdiploidy (> 50 chromosomes) is a favorable prognostic feature in childhood ALL.33 A significantly lower frequency of hyperdiploidy in the ALL-DS population was seen in this cohort, as in others.13-15 A decreased prevalence of hyperdiploidy may contribute to poorer outcome in the ALL-DS population.
Specific chromosomal translocations, including t(9;22) and t(4;11), are associated with adverse outcomes in childhood ALL.34 No adverse translocations occurred in the ALL-DS population, in contrast to an incidence of 8.4% in the ALL-NDS population. While t(9;22)(q34, q11)35 and t(4;11)(q21, q23)36 are each observed in approximately 5% of childhood ALL, others have similarly described an absence13,15 or decreased prevalence14 of adverse translocations in ALL-DS populations. Adverse translocations are frequently associated with high-risk features such as an elevated WBC at diagnosis; thus, their absence in the ALL-DS cohort could contribute to a better outcome in the HR subset of the ALL-DS population.
TEL-AML1 rearrangement is the most frequent genetic abnormality identified to date in childhood ALL and is generally associated with a good prognosis.37,38 TEL-AML1 rearrangements appear to be uncommon in the ALL-DS population; in 1 report, blasts from 11 ALL-DS patients had no TEL-AML1 rearrangements.39 Although only 1 case of TEL-AML1 rearrangement was identified among 16 ALL-DS patients in the Medical Research Council United Kingdom Acute Lymphoblastic Leukemia (MRC UKALL) trials,15 this finding lacked statistical significance. A decreased incidence of TEL-AML1 rearrangements in the ALL-DS population would be expected to contribute to poorer overall and event-free survivals. We were unable to perform TEL-AML1 analysis in the present study, due to limited availability of leukemic samples. The increased frequency of hyperdiploidy, absence of adverse translocations, and absence of ALL-DS in children less than 1 year of age demonstrate the existence of important biologic differences between the ALL-DS and ALL-NDS populations, which could help explain some of the observed differences in survival.
We observed that children with ALL-DS on CCG trials were significantly less likely to attain remission by day 28. This finding is consistent with a previous CCG report3 but differs from POG, BFM, and UKALL studies,12,14,15 in which ALL-DS patients were equally likely to attain remission. Failure to attain remission in a higher percentage of patients may be attributable to differences in the incidence of known prognostic factors, such as hyperdiploidy or TEL-AML1 rearrangements, common to all childhood ALL, but may also be in part attributable to factors that are unique to the blasts of ALL-DS patients, such as differences in sensitivity to, or metabolism of one or more chemotherapeutic agents. The German Berlin-Frankfurt-Munster (BFM) trials showed a nonsignificant trend in ALL-DS patients for favorable initial treatment response to prednisone, suggesting ALL-DS blasts are not less sensitive to steroids.14 Taub et al reported that leukemic myeloblasts from patients with DS are more sensitive to both cytosine arabinoside and daunomycin40,41 ; these findings may account in part for the increased survival of patients with DS-AML.25 Although leukemic blasts from patients with ALL-DS were not included in their study, Taub et al point out that trisomy 21 in pediatric B-precursor ALL is associated with a favorable prognosis,41 implying that this may be due in part to increased drug sensitivity. Anthracyclines were not used in CCG SR-ALL induction regimens but were included in CCG HR-ALL, BFM, and UKALL protocols; increased sensitivity of ALL-DS blasts to anthracyclines could contribute to the increased risk of remission induction failure seen only in patients with DS on CCG SR-ALL protocols. However, only 3 patients with ALL-DS demonstrated remission induction failure, and it is difficult to draw firm conclusions regarding differences in induction remission rates between ALL-DS and ALL-NDS patients in the current study because of the sample size limitation.
We identified an overall improvement in event-free survival for ALL-DS patients enrolled on CCG trials in the recent era (1989-1995; CCG-1800 series) as compared with earlier era (1983-1989; CCG-100 series). However, upon analyses of cohorts by risk group within these treatment eras, we demonstrated that the standard-risk patients with ALL-DS continued to have inferior outcomes, and the high-risk patients continued to have comparable outcomes compared with ALL-NDS patients, regardless of the treatment era.
ALL-DS patients are more likely than ALL-NDS patients to develop treatment complications such as mucositis and hematologic toxicities,11,12,42-45 both of which increase susceptibility to infection. The higher prevalence of mucositis in ALL-DS patients has not been described in AML-DS patients, likely due to the enhanced sensitivity of ALL-DS patients to methotrexate,42,43,45 an agent not routinely used in AML therapy. In addition to an increased propensity for developing mucositis after receiving methotrexate, DS patients have altered immune function, as shown by reduced B lymphocyte numbers and T lymphocyte proliferative responses compared with controls.46,47 The underlying immunodeficient state in ALL-DS patients may contribute to an increased susceptibility to infections. Unfortunately, we could not evaluate our cohort for mucositis, infectious complications, or other specific toxicities due to limitations related to toxicity-related data gathered on these patients.
Robison et al3 observed a significantly higher risk of death during induction for ALL-DS patients compared with ALL-NDS patients, with most deaths attributable to infections. Levitt et al10 described a higher prevalence of deaths due to infection during induction therapy in ALL-DS patients treated with 1- or 2-drug induction therapy (5%) and in those who had more intensive induction therapy (13%), in contrast to the ALL-NDS population (0.5%). Deaths during induction among patients with ALL-DS in our series were attributable to massive gastrointestinal bleeding, overwhelming infection, and acute cardiac failure.
Children with DS are known to have a significantly high prevalence of congenital cardiac malformations,48 which could potentially contribute to an increased risk of cardiac complications after anthracycline administration. Although we were unable to evaluate cardiac toxicities in the present study, prior studies of ALL-DS patients have not identified cardiac toxicity in the ALL-DS population as a significant cause of mortality.3,10,11,14
This analysis of 179 children with DS treated for ALL in a cohort of 8447 children with ALL diagnosed between 1983 and 1995 and treated on CCG therapeutic protocols represents the largest cohort described to date. Multivariate analysis demonstrated DS to be a significant adverse risk factor for outcome independent of other recognized risk factors; subset analysis by risk stratification showed that this adverse risk was restricted to the standard-risk ALL-DS population. Unique clinical and biologic features were identified in the ALL-DS patients in our study. Hyperdiploidy was less common in the ALL-DS population, and chromosomal translocations associated with adverse outcome were absent. ALL-DS patients less than 1 year of age were absent from our cohort. On the other hand, the details regarding presence or absence of TEL-AML1 alterations were not known in this cohort. However, none of these clinical or biologic features satisfactorily account for the poorer outcomes observed in this cohort.
Our results suggest that intensive therapy in ALL-DS patients with high-risk features results in outcomes comparable with those in patients with ALL-NDS; delivery of intensive therapy is thus warranted to high-risk ALL-DS patients, but careful attention must be paid to the mucositis, infections, and other complications that are more common in these children. Dose reductions in chemotherapeutic agents in the ALL-DS population due to concerns of anticipated toxicities may adversely affect outcome and should be avoided in the absence of demonstrated chemotherapy intolerance. Factors underlying the inferior outcome of ALL-DS patients with standard risk features need to be explored in greater detail, and new therapeutic strategies need to be developed, in the context of prospective clinical trials, to address the poorer outcomes of standard-risk ALL-DS patients. Therapies that improve event-free survival while minimizing toxicities in ALL-DS patients will require new insights into the unique pathogenesis and biology of ALL in this population.
Future studies of children with ALL-DS should focus on better defining the unique biologic features of these patients' leukemic cells. Analysis of TEL-AML1 alterations in ALL-DS patients will determine this molecular lesion's role in pathogenesis. Microarray analysis has identified biologically distinct categories of ALL and AML not defined by conventional biologic means such as cytogenetics49,50 and could offer a powerful tool in better understanding the unique biology of the leukemias that occur in DS.
Prepublished online as Blood First Edition Paper, August 18, 2005; DOI 10.1182/blood-2003-10-3446.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal