Comment on Lo et al, page 4159
Genesis of platelet dense granules has been studied largely by characterization of molecular defects in patients with syndromes that include dense granule defects. In contrast, little is known about α-granule formation. Lo and colleagues now describe an inherited α-granule defect that demonstrates a requirement for VPS33B in α-granule biogenesis.
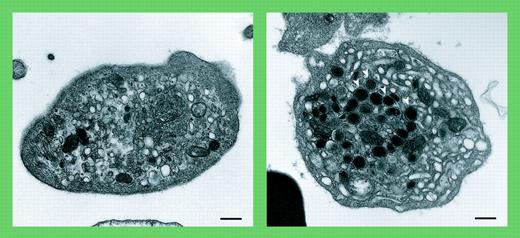
Arthrogryposis, renal dysfunction, and cholestasis (ARC) syndrome is a rare autosomal recessive condition characterized by death in the first year of life. In addition to the maladies that give this syndrome its name, patients with ARC syndrome suffer from a bleeding diathesis. Platelets from patients with ARC syndrome are large and lack α-granules.1 Lo and colleagues further characterize these platelets as not only being deficient in α-granules, but also having increased dense granules. The authors also distinguish ARC platelets from those observed in gray platelet syndrome. For example, ARC platelets demonstrate negligible P-selectin by immunoblot analysis and an absence of α-granule membranes by electron microscopy. In the gray platelet syndrome, P-selectin and glycoprotein IIb-IIIa (GPIIb-IIIa) are observed on membranes of abnormal precursor granules.2 Thus, ARC platelets represent a unique platelet phenotype.
Absent α-granules in platelets from patients containing mutations in VPS33B. See the complete figure in the article beginning on page 4159.
Absent α-granules in platelets from patients containing mutations in VPS33B. See the complete figure in the article beginning on page 4159.
A great incentive to study platelets from patients with ARC syndrome is that the genetic lesion responsible for the phenotype has been defined. The disorder is caused by mutations in the VPS33B gene.3 All subjects studied by Lo et al carried mutations in this gene. The VPS33B gene product is a member of the Sec-1/Munc18 family, which is involved in vesicular trafficking. Sec-1/Munc18 family members modulate membrane fusion events via their strong interaction with members of the syntaxin family of soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE) proteins. Functions for Sec-1/Munc18 family members in platelet membrane fusion events have previously been shown. One such example is VSP33A, a homolog that shares 31% identity to VPS33B. VSP33A-deficient mice demonstrate a bleeding phenotype and a dense granule deficiency.4 Munc-18a, b, and c are also members of the Sec-1/Munc18 family and have been shown to regulate platelet granule secretion.5,6 However, a role for VPS33B in platelet membrane trafficking had not previously been studied.
Knowing the molecular identity of the platelet defect, the investigators were able to make several observations regarding the role of VPS33B in α-granule biogenesis. Their immunofluorescence studies of normal megakaryocytes demonstrate that VPS33B colocalizes with α-granule precursors. In contrast, VPS33B does not show significant colocalization with dense granule precursors. The investigators also show that VPS33B is found in megakaryocytes, but not in mature platelets. This result demonstrates that VPS33B is not involved in platelet granule secretion (or other functions of mature platelets).
Although these studies do not conclusively demonstrate that VPS33B is involved exclusively in α-granule and not dense granule biogenesis, they raise this possibility. The prominent role of VPS33B in α-granule biogenesis may enable identification of protein complexes (as described in dense granule biogenesis) that mediate α-granule biogenesis. Such information could lead to the generation of animal models to directly compare and contrast the respective roles of α-granule and dense granules in thrombus formation and inflammation. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal