Abstract
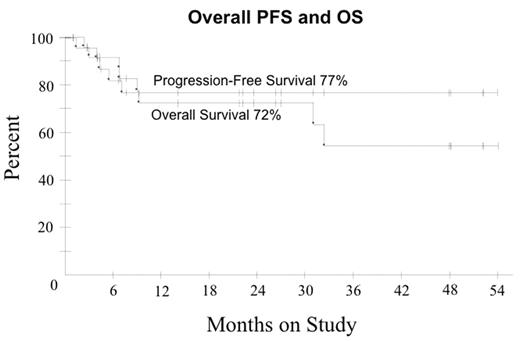
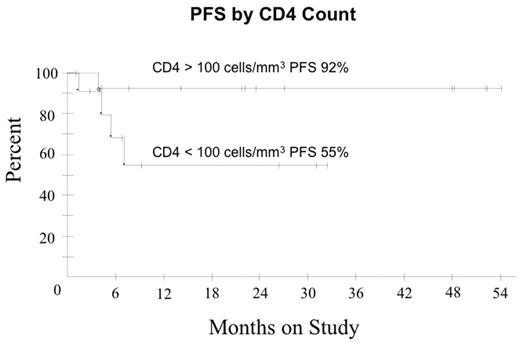
In ARL, the addition of Rituximab ® to CHOP chemotherapy may improve tumor response but the benefit appears offset by increased infectious deaths (Kaplan et al. Blood 2005; May 24: Epub). We hypothesized R with DA-EPOCH may enhance tumor kill, enabling fewer treatment cycles and lowering toxicity compared to DA-EPOCH alone. Pts received DA-EPOCH-R (in mg/m2/d etoposide 50, vincristine 0.4 and doxorubicin 10 all CIV d 1–4; and in mg/m2 cyclophosphamide 750 IV day 5, prednisone 60 po days 1–4 and rituximab 375 IV d 1, 5; and G-CSF sc d 6–15). Prophylactic IT MTX x 6 was administered and HAART was discontinued on all cycles. Cyclophosphamide was adjusted based on ANC nadir. Response was assessed by CT and PET and pts received 1 cycle beyond CR for a minimum of 3 cycles. Characteristics of 27 pts include median (range) age 41 (9–61) years; IPI 3 (0–4); ECOG PS 1 (1–4), CD-4 212 (0–674) cells/mm3; HIV viral load 58,700 (0–6,080,000) RNA copies/mL; male sex 22 (81%); LDH > N 19 (70%); stage IV 18 (67%) and histology DLBCL 25 (93%) and Burkitt lymphoma 2 (7%). Of 24 evaluable pts (2 NE, 1 TE), median (range) cycles is 3 (3–5) with CR/Cru in 21 (88%) and PR 1 (4%) pts. At 25 mos median follow-up, PFS and OS are 77% and 72%, respectively. Shown below are PFS and OS for the entire group and PFS in pts with CD4 > and < 100 cells/mm3. In pts with CD4 > and < 100 cells/mm3, OS was 92% and 48%, respectively, indicating a relatively low probability of non-tumor related deaths at 2 yrs among pts in remission. IPI did not impact OS or PFS. The predictive value of early PET (after cycle 2 or 3) for progression was evaluated in 21 pts in remission. None of 11 pts with a negative PET progressed (100% negative predictive value) whereas only 2 of 10 pts with a positive PET progressed (20% positive predictive value). Toxicity on 81 cycles included ANC < 500/mm3 on 33 (41%); platelets < 50000/mm3 on 20 (25%) and; fever/neutropenia on 27 (33%). No treatment related deaths occurred. Short course DA-EPOCH-R is highly effective and tolerable in all subgroups of ARL with significantly fewer treatment cycles (median 3 versus 6 cycles) compared to DA-EPOCH alone. CD4 cells decreased a median of 42 cells/mm3 (range +271 to − 320) by end of therapy with DA-EPOCH-R compared to 189 cells/mm3 (range +19 to −973) with DA-EPOCH alone. While a negative PET scan reliably predicted a disease-free state, a positive PET scan only predicted treatment failure in 20% of pts in remission. Although this study was not designed to assess the benefit of rituximab, a comparison to DA-EPOCH (
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal