Abstract
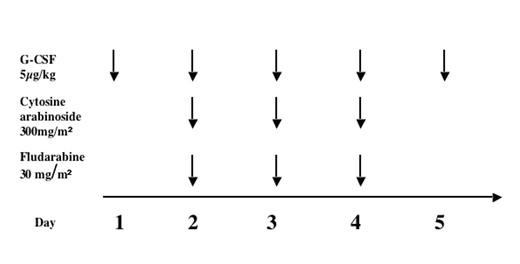
Fanconi anemia (FA) patients have a 33% cumulative incidence of AML by 40 years of age (Kutler et al, 2003). Chemotherapy treatment of FA patients with MDS or AML is challenging because of constitutional sensitivity to DNA-damaging agents. Severe toxicity and marrow aplasia without hematological recovery have been described, largely in case reports of children given chemotherapy prior to diagnosis of FA. The definitive treatment for hematological malignancy in FA is stem cell transplant (SCT). However, transplant in patients with overt AML has a poor prognosis and also a donor may not be immediately available. Relapse post-SCT is usually fatal. Between 2002 and 2005 we treated 6 children with FA and AML or hypercellular MDS with monosomy 7, referred to the Fanconi Anemia Comprehensive Care Clinic at Cincinnati Children’s Hospital, with a uniform chemotherapy protocol (“mini-FLAG”).
Clinical characteristics of patients, toxicity and efficacy of mini-FLAG are shown below: 3 patients belonged to complementation group A and one each to group D1, J and F. Overall, mini-FLAG was well tolerated with no mucositis or organ toxicity. Two of the three patients with leukemia achieved clearance of blasts and cytoreduction was attained in those with MDS. In all cases, patients proceeded to SCT once cytoreduction was achieved so reconstitution of normal hematopoiesis and durability of response could not be assessed.
| Pt # . | Age / Gender . | Marrow cytogenetics . | Toxicity of mini-FLAG . | Marrow morphology pre-mini-FLAG . | Marrow morphology after mini-FLAG . |
|---|---|---|---|---|---|
| 1 | 16.3yrs/M | 45,XY, t(2;3) | No organ toxicity | AML; 51% blasts | No residual blasts; markedly hypocellular marrow |
| 2 | 14.2yrs/F | 47,XX, t(6;11) | No organ toxicity | AML; 80% blasts | Hypoplastic; <5% blasts |
| 3 | 2yrs/M | Complex karyotype including add(1)(q21),add | No organ toxicity; reversible skin rash with Ara-C | AML; 75% blasts | Blasts reduced to 36%; marrow blasts cleared with daunomycin + Ara-C |
| 4 | 13.7yrs/F | 46,XX,del(7) | No organ toxicity | CMMoL; hypercellular | Normocellular marrow; morphology still CMMoL |
| 5 | 8.5yrs/M | 46,XY,der(2)t(2;3)(q3?6;q?),der (7)del(7)(p11.2) | No organ toxicity | MDS; hypercellular; prominent abnormal monoblasts | Hypocellular; minimal dysplasia |
| 6 | 10.6yrs/F | 45,XX,-7 | No organ toxicity | Mild dysplastic changes | Hypocellular; Monosomy 7 in 13% of cells |
| Pt # . | Age / Gender . | Marrow cytogenetics . | Toxicity of mini-FLAG . | Marrow morphology pre-mini-FLAG . | Marrow morphology after mini-FLAG . |
|---|---|---|---|---|---|
| 1 | 16.3yrs/M | 45,XY, t(2;3) | No organ toxicity | AML; 51% blasts | No residual blasts; markedly hypocellular marrow |
| 2 | 14.2yrs/F | 47,XX, t(6;11) | No organ toxicity | AML; 80% blasts | Hypoplastic; <5% blasts |
| 3 | 2yrs/M | Complex karyotype including add(1)(q21),add | No organ toxicity; reversible skin rash with Ara-C | AML; 75% blasts | Blasts reduced to 36%; marrow blasts cleared with daunomycin + Ara-C |
| 4 | 13.7yrs/F | 46,XX,del(7) | No organ toxicity | CMMoL; hypercellular | Normocellular marrow; morphology still CMMoL |
| 5 | 8.5yrs/M | 46,XY,der(2)t(2;3)(q3?6;q?),der (7)del(7)(p11.2) | No organ toxicity | MDS; hypercellular; prominent abnormal monoblasts | Hypocellular; minimal dysplasia |
| 6 | 10.6yrs/F | 45,XX,-7 | No organ toxicity | Mild dysplastic changes | Hypocellular; Monosomy 7 in 13% of cells |
Three children survive; one died of relapsed leukemia 4 months post-SCT and 2 of complications of SCT. These data show that mini-FLAG can be used with minimal toxicity and significant but heterogeneous responses in children with FA. A future prospective multi-center study is needed to optimize use of chemotherapy in FA and determine the effect of pre-transplant chemotherapy on outcomes of SCT.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal