Abstract
As2O3 is an effective therapeutic agent in the management of acute promyelocytic leukemia (APL). Concerns have been raised about its potential cardiac and hepatotoxicity. We retrospectively analyzed the hepatotoxicity profile in newly diagnosed patients treated at our center, its impact on survival and also studied the effect of genetic polymorphisms on its incidence. Polymorphisms that were studied included some members of the GST family of genes (GSTM1, GSTT1and GSTA1) and that of the MTHFR gene (MTHFR C677T and MTHFR A1289C), these could potentially have a role in the bio-transformation of As2O3 and in handling reactive oxygen species generated by it.
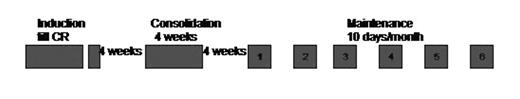
Between January 1998 and April 2005, 83 newly diagnosed patients with APL were treated with a regimen of single agent As2O3 in induction, consolidation and maintenance at doses of 10 mg/day for adults and 0.15mg/kg/day for the pediatric population.
There were 7 early deaths (6 IC bleeds, 1 sepsis), these patients were not evaluable for toxicity. Liver function tests (LFT) were done weekly in induction, once in 2 weeks in consolidation, prior to every course of maintenance and at three monthly intervals on follow up.
Of the 76 evaluable patients in 46 (60.5%) there was no evidence of hepatotoxicity, grade 1, 2, 3 and 4 toxicity (NCI-CTC v2.0) was seen in 14(18.4%), 8(10.5%), 4(5.3%) and 4(5.3%) patients respectively. Of the 4 patients with grade 4 toxicity; in 3 there was documented acute hepatitis B infection. Onset of hepatotoxicity occurred in induction in 19 (63.3%), during consolidation in 3 (9.9%), during maintenance in 6 (20%) and following completion of therapy in 2 (6.6%). Of the 30 (39.5%) patients who developed hepatotoxicity in the majority the prominent abnormality was transaminitis (29 – 96.6%), it was transient (<4 weeks duration) in 20 (66.7%) and resolved on continuing As2O3 in 20 (66.7%). For all patients with grade 3 – 4 toxicity As2O3 was withheld till the LFT had normalized. One patient with grade 2 toxicity died on day 42 secondary to uncontrolled sepsis. Two patients have persisting LFT abnormality post completion of therapy (one each grade 1 and grade 2). There were no cases of acute hepatic failure or mortality attributable to hepatic toxicity.
There was a significant association of hepatotoxicity with the MTHFR A1298C polymorphism (p=0.033). There was no statistical correlation of hepatotoxicity and null phenotype of GSTT1 or GSTM1 or with polymorphisms of GSTA1and MTHFR C677T. Occurrence of hepatotoxicity had no impact on OS in this group of patients (Log rank test p=0.666). In patients who developed hepatotoxicity EFS was superior to those that did not (97% vs 73%) though this did not reach statistical significance (p=0.116).
Conclusion: The hepatotoxicity profile of single agent As2O3 as used in this regimen in the treatment of newly diagnosed cases of APL is mild and transient in the majority of patients. Its occurrence correlates with presence of the MTHFR A1298C polymorphism and it does not have an adverse impact on EFS or OS.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal