Abstract
Most patients (pts) transplanted with active myeloid leukemias will relapse after HSCT. Intensity of the conditioning regimen is an important component of disease control. We hypothesized that GO could safely increase the anti-disease activity of FM, and investigated this hypothesis in the trial reported here.
Patients and Methods: Objective: to determine the dose of GO with the lowest toxicity (tox), and the highest response probabilities. Tox: grade 3–4 organ tox, engraftment failure or early death (ED; first 30 days post HSCT). Response was defined as no tox, engraftment, and remission (CR) on day +30. Trial was designed with GO doses of 4, 6 and 9 mg/m2 but given tox observed at 4mg/m2, doses 6 and 9 mg/m2 were not used and dose 2mg/m2 was added. Eligible were pts aged 12–75 years, not candidates for high-dose regimens or with high-risk CD33+ disease. Treatment: GO 2 or 4mg/m2 day -12, F 30mg/m2 (days -5 to -2), M 140mg/m2 (day -2); HSCT day zero. ATG was added in unrelated (UD) HSCT. Graft-versus-host disease (GVHD) prophylaxis: tacrolimus and mini-methotrexate (5mg/m2 day+1,+3,+6,+11). GO was given on day -12 to minimize probability of delaying engraftment.
We treated 52 pts, median age 53 yrs (13–72), with AML (n=47), MDS (n=4) or blast crisis CML(n=1). Disease status at HSCT: CR (n=3),induction failure (n=15), first/second relapse (n=33), untreated MDS (n=1). 18 pts had a previous HSCT (allogeneic, n=11 or autologous, n=7). At study entry, median number of bone marrow blasts was 17% (0–95%); 33% of the pts had circulating blasts, 4 pts were FLT3 positive and 38% had poor prognosis cytogenetics. Median blast CD33 expression: 89% (22.5–99.6). Donors: related (n=33) or UD (n=19). Stem cell source was peripheral blood (n=43) or bone marrow (n=9).
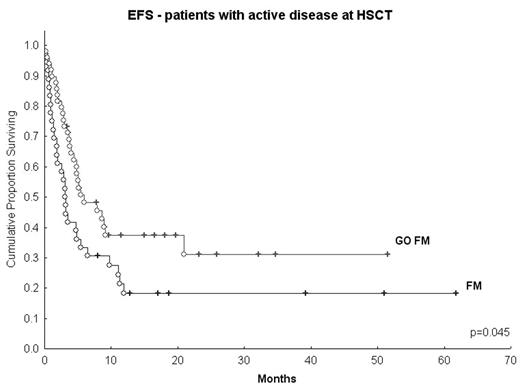
Results: Median day 30 donor cell chimerism was 100%. Median time to ANC> 500/mm3 was 13 days. Eight pts received GO at 4 mg/m2; 2 died early (due to pneumonia and renal failure) and 2 developed gd III gastro-intestinal and renal tox. 44 pts were then treated at 2 mg/m2: 3 pts died of regimen-related tox (2 ED due to pulmonary bleeding and sepsis, and 1 death within the first 100 days due to pneumonia, renal failure/TTP/HUS). 100-day treatment-related mortality (including 2 deaths due to aGVHD and 1 due to infection) was 15% (n=8). Tox included reversible gd III-IV bilirubin (n=2), transaminase elevation (n=5), moderate hepatic VOD (n=1) and gd I-II GI tract (n=39). CR rate was 90%; 1 pt failed to respond. Gd II-IV and III-IV aGVHD rates were 44% and 23%, respectively, and 58% developed cGVHD. Median follow-up is 9 months (2.5–36) for surviving pts (n=29); 17 pts have relapsed. Median event-free-survival (EFS) is 3.8 mos. Median EFS of a historic control group treated with FM140 (n=36) was 2.2 mos (Figure). Tox of GOFM is similar to that documented by our group with FM140 mg/m2 in a somewhat better prognosis population.(
Conclusion: GO 2mg/m2 can be safely added to FM, and may improve the anti-leukemic efficacy of the regimen.
EFS - patients with active disease at HSCT
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal