Abstract
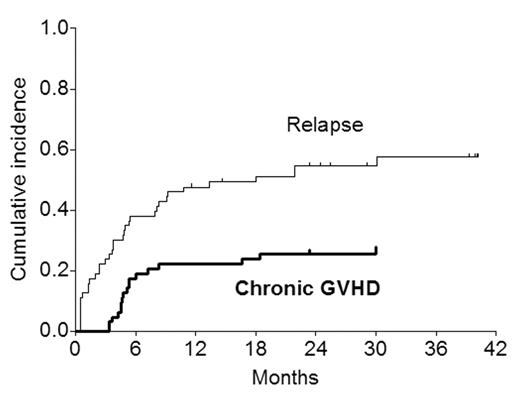
Since graft-vs-tumor effects are the mainstay of sustained disease control after submyeloablative (mini) allogeneic transplantation, rapidly-progressive malignant disease may not be curable with SMAT. Figure 1 shows the cumulative incidence of chronic GVHD and relapse (n=35) in 63 patients undergoing mini-allografts for hematologic malignancies (acute leukemia, lymphoma/CLL, myeloma).
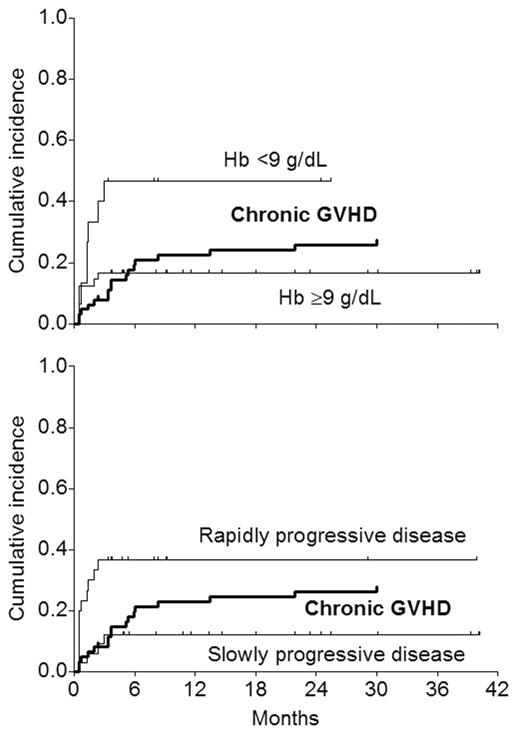
The pattern suggests that a substantial number of relapses occur before chronic GVHD and associated GVT get established. The purpose of this analysis was to see if patients at risk of early relapse (relapse within 100 days; n=15 in this series) could be identified based on pre-transplant characteristics (diagnosis, chemosensitivity, Hb <9, platelets <100, abnormal LDH, albumin <3, performance status). In Cox analysis, the only factor predictive of early relapse was Hb <9 at the time of transplant (7 of 15 vs 8 of 48 with Hb ≥9; RR=3.1; P=0.029). The patients were also retrospectively classified by their physicians to have “slow” (n=33) or “rapid” (n=30) disease pre-transplant based on clinical impression. 11 early relapses occurred in patients with “rapid” disease and 4 in those with “slow” disease. When disease tempo was introduced in the Cox analysis as an additional variable, it was of borderline significance (P=0.051); with Hb <9 remaining as the sole predictive factor.
The curves showing the temporal relationship between chronic GVHD and early relapse based upon Hb or disease tempo suggest that patients with Hb ≥9 or those with “slow” disease stand to benefit more than the others from graft-vs-tumor effects. Accordingly, restriciting mini-allografts to patients with Hb ≥9 or those with “slow” disease would be expected to improve outcome in terms of disease-free survival. However, neither of these two factors is particularly satisfactory: Hb <9 identifies only half the patients relapsing early, and the subjective assessment of disease tempo by the treating physician, while identifying a greater proportion of patients at risk of early relapse, excludes almost half the patients from the allograft. These data suggest that studies are needed in larger groups of patients to develop more robust (sensitive as well as specific) criteria to identify patients at risk of early relapse after mini-allografts. Since such patients are unlikely to benefit from a standard mini-allograft procedure, they may be better suited for alternatives such as conventional-intensity allografts, tandem autograft and mini-allograft procedures, or novel immunotherapeutic approaches applied very early after a mini-allograft.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal