Abstract
Background: we have shown in a previous study that approximately 30% of patients may experience poor graft function (PGF) following an allogeneic hemopoietic stem cell transplant (HSCT); it is uncertain whether a boost of donor stem cells is beneficial in terms of trilineage recovery (TLR) and transplant related mortality (TRM).
Aim of the study: To compare infusion of boost donor stem cells in patients with primary PGF (who failed to achieve sustained graft function) and secondary PGF (patients who developed PGF after complete hematologic recovery).
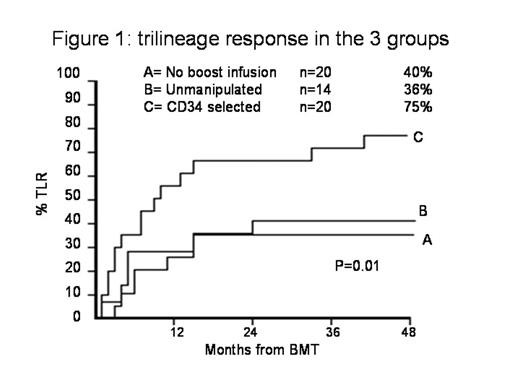
Methods: we have attempted to rescue PGF using stem cells from the same donor in 54 patients affected by hematologic malignancies: 20 patients received no further infusion (group A), 14 received a boost of unmanipulated marrow or blood cells from the original donor, without further conditioning (group B), and 20 received donor cells after CD34 selection without conditioning (group C).
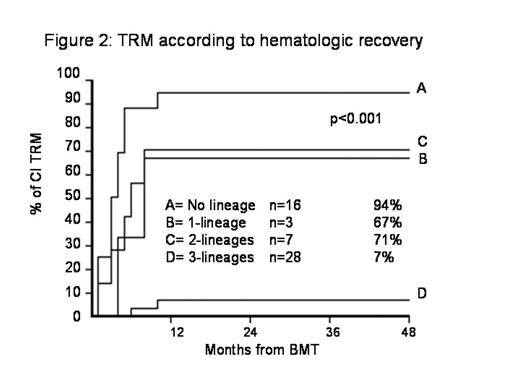
Results: Respectively 40%, 36% and 75% of patients in groups A, B and C obtained a trilineage recovery (p=0.02). TLR was more frequent in secondary PGF (69%) vs primary PGF (36%) (p=0.09), and more frequent in recipients of PB boost donor cells (p=0.02). There was no effect of donor type on hematologic recovery. TRM was 55%, 64%, 25% respectively in groups A, B and C (p=0.06) and was highly correlated with TLR (p=0.00001). PGF was the primary cause of death in 30%, 21%, 10% in the 3 groups, graft vs host disease in 5%, 36%, 10%.
Conclusion: The study indicates that a boost of CD34 selected PB donor cells is associated with a high chance of trilineage recovery and low risk of acute GvHD. A boost of unmanipulated donor cells does not seem to offer an advantage over no infusion. TRM is lower when using PB CD34 selected cells.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal