Abstract
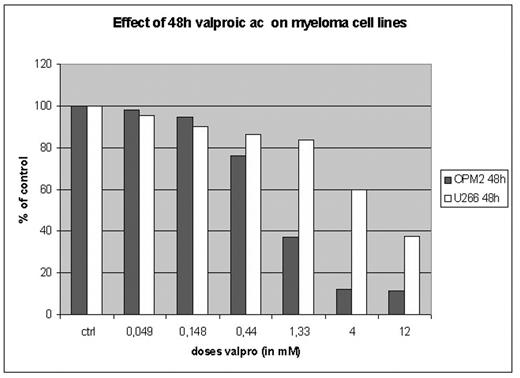
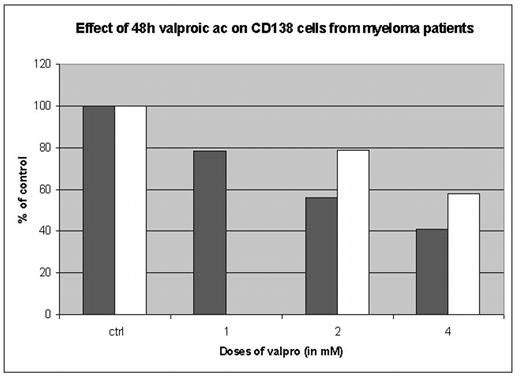
Multiple myeloma is, despite the emergence of new treatment strategies in recent years, still a lethal disease in 2005. Over the last several years, significant insights into the dysregulation of various signal transduction pathways of MM have emerged and have evolved the development of new agents. Histone deacetylase inhibitors as valproic acid are compounds that inhibit HDACs enzymes that, in conjunction with histone acetylases, regulate the acetylation state of histones and, by extension, the conformational status of chromatin. VPA is clinically known to be used in treatment of different types of epileptic disease. Effects and signal transduction pathways of cell death have been studied in cells harvested and purified from routine bone marrow aspirates of several patients with multiple myeloma, as well as on myeloma cell lines (OPM2, U266) treated with a physiologic dose range of valproic acid (1–4 mM). We observe significant in vitro toxicity starting at doses of 1 mM for 24h in cell lines as well as in patient cells
using an XTT based cytotoxicity assay. The question we adressed was the mechanisms by which the MM died. Flow cytometric analysis with PI / Annexin V staining does not show apoptosis features, nor do TUNEL staining or DNA fragmentation assays, suggesting non activation of the intrinsic apoptosis pathway. In contrast, cell morphology of treated cells stained with May-Gruenwald-Giemsa staining show increase in multinucleated giant cells. Multinucleation has often been described in cells which die through mitotic catastrophe. Further experiments exploring this hypothesis will be conducted.
Effect of 48h valproic ac on myeloma cell lines
Effect of 48h valproic ac on CD138 cells from myeloma patients
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal