Abstract
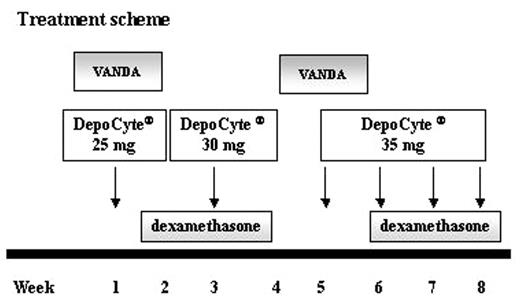
Leptomeninges are a frequent site of recurrence in childhood acute lymphoblastic leukemia (ALL), often difficult to eradicate for the limited drug penetration through the blood-brain barrier. The main IT anticancer agents have a limited efficacy for their rapid metabolism and low cytotoxic liquor concentrations. Recently a new liposomal formulation of cytarabine (Depocyte®), encapsulated into multivesicular lipid particles, is under investigation in lymphomatous meningitis for its property of slow release of cytarabine into the CSF. We evaluated the efficacy and toxicity of Depocyte® in a child with repeated meningeal relapses. A 8 years-old boy, with hyperleukocytosic T-ALL was enrolled in the AIEOP high risk protocol after first diagnosis and presented with CSF hemorrhage, seizures and coma at day +22 which required intensive care. Besides, he showed VCR toxicity in two different occasions with VII cranial nerve paresis. Eight months after initiation of the front-line protocol, during the re-induction treatment, he presented with a meningeal relapse and a second line AIEOP protocol was started. At month +11 from disease onset, he showed diplopia, eyelid ptosis, oculomotor nerve paresis, intense headache, vomit and vertigo. Lumbar puncture was positive for T-blasts and the MRI showed an enlargement of bilateral optic nerves and persistent malacic area as a residual of the previous hemorrhage. A second CSF relapse combined with a bone marrow recurrence was diagnosed. He started on a rescue protocol with VANDA modified without L-ASP (ARA-C 2 gr/m2 twice a day for 2 days, DMZ 20 mg/m2 x 5 days, VP-16 150 mg/m2 x 3 days, MITO 8 mg/m2 x 2 days) associated to IT administration of Depocyte® at scalar doses (T1=25 mg, T2=30 mg) every two weeks for the first two doses. Because of the persistence of blasts at T3, IT Depocyte® was changed to be administered weekly for 4 injections (T3–T6), at 35 mg/dose, associated with a second cycle of VANDA. Arachnoiditis prophylaxis with dexamethasone at 0.15 mg/kg/day was performed. At T4, the liquor was completely negative and neurological and ocular symptoms had disappeared. The MRI showed complete resolution of optic nerve involvement. No adverse effects were reported. A complete hematological and meningeal remission has been achieved and a cranial radiotherapy as consolidation of CSF remission is ongoing. In this single experience, we observed the efficacy of weekly administration of liposomal ARA-C to control leptomeningeal metastasis and particularly optic nerve involvement. We suggest that the weekly administration of Depocyte®, combined with conventional chemotherapy, should be further evaluated as a potential treatment schedule in pediatric patients. The drug was well tolerated and no adverse events were reported. Further investigations are warranted to evaluate the efficacy and tolerability of Depocyte® in the pediatric setting.
Treatment scheme
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal