Abstract
Introduction: In patients with congenital FVII deficiency and positive bleeding history surgical procedures should be covered by an sufficient substitution therapy of FVII concentrate. For the first three postoperative days trough level of at least >0,2U are recommended. We report haemostasis management of a complex thoracic surgery procedure in a 14 year old boy with FVII deficiency and diagnosis of a mucoepidermoid bronchogenic carcinoma of the left upper lobe. Extended thoracic surgery without extra corporal circulation was performed as a lobectomy of the left upper lobe including extended preparation of lymphnodes.
Preoperative Lab:
Platelets 285000/μl, INR 1,6, aPTT 28s, fibrinogen 239 mg/dl, FVII 0.33IU
Because of positve bleeding anamnesis, the extended surgical procedure and possibility of further extension of the planned procedure we decide to cover surgery by factor treatment to be on the safe side though FVII activity was 0.33IU. Because of virus safety and safety profile we decide to give rFVIIa (NovoSevenR) as bolus injections. Frequency of treatment was chosen according to regular lab controls and clinical course.
Haemostasis management during surgery:
Preoperative rFVIIa dosage: 30 μg/kg BW, given immediately before skin cut as a bolus. Intraoperativly was no significant bleeding and no transfusion requirements though it was a complex and extended procedure.
Haemostasis management postoperatively:
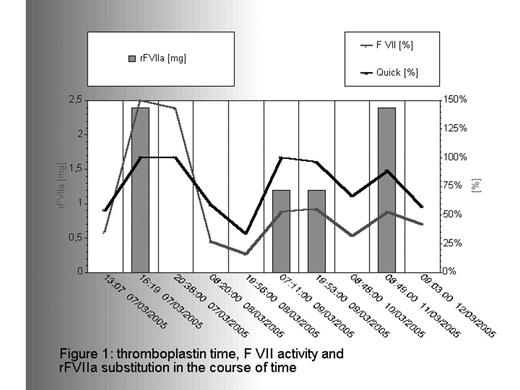
Regular control of FVII activity and Hb was done every 6 hours to decide if substitution therapy is required. In the early phase after surgery FVII activity remained >1.0 IU, so additional factor treatment was not necessary. Hb remained stable in normal range. 24h after surgery FVII activity fell to 0.16IU. Substitution was done with dosage of 15μg/kgBW rFVIIa as a bolus (NovoSevenR). Frequency: once daily, duration: 2 days Clinical course: no bleeding complications. For remove of drainges a last bolus of 30μg/kg was given. Residual FVII activity: >0,2IU in all regular controls during the first three days.
thromboplastin time, F VII activity and tFVIIa substitution in the course of time
thromboplastin time, F VII activity and tFVIIa substitution in the course of time
Results:
Bleeding/Transfusion requirements: No bleeding complications or wound healing complications occurred, no blood products has been transfused.
Adverse events: no adverse events, no thrombembolic events and no inhibitor development.
Conclusions: Regular control of FVII activity and clinical bleeding signs led to an efficient use of factor concentrates. rFVIIa given as bolus in FVII deficiency seem to be a save and efficient therapy for haemostasis management in complex thoracic cancer surgery.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal