Abstract
Purpose: From May 2003 to March 2005, 37 patients with haematological malignancies were transplanted with peripheral blood stem cells after nonmyeloablative conditioning with fludarabine 30 mg/m2 i.v. once daily on day −4 to −2 and 200 cGY of total body irradiation on day 0. Post-transplant immunosuppression consisted of oral cyclosporin 12.5 mg/kg/day and mycophenolate mofetil 30 mg/kg/day. Three patients were not eligible for follow-up due to lack of informed consent or informative markers for chimerism measurement. The purpose of this study was to evaluate kinetics of early chimerism of CD4+, CD8+, and, CD15+ cells immediately post transplant and furthermore to identify factors associated with rejection of graft.
Methods: Blood samples were collected post transplant on day +1–3, +5–7, and then weekly. Samples were separated with immunomagnetic beads and DNA was salt extracted. Chimerism analysis was performed by automated high-resolution real-time quantitative PCR based on short insertion or deletion polymorphisms or single nucleotide polymorphisms.
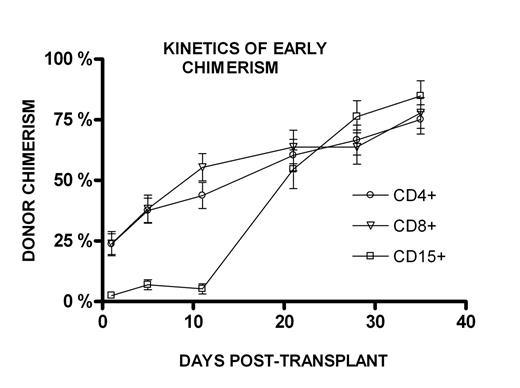
Results: The kinetics of CD15+ chimerism differed from that of CD4+ and CD8+ chimerism (Figure). CD15+ donor chimerism was low with a median of 0.9% donor cells on day +1–3, and remained low until around day +14. Subsequently the donor chimerism increased rapidly towards 100%. The CD4+ and CD8+ donor chimerism in contrast started higher with a median of 16.3% and 15.1% respectively on day +1–3, increased slowly, and took months to reach 100%. CD4+, CD8+ or CD15+ donor chimerism on day +1–3 did not predict later development of acute graft versus host disease. Two patients had primary rejection of the donor stem cells, while four patients had secondary rejection. The patients with primary rejection had no measurable donor chimerism in any cell lineages from day +1–3. No patient with CD15+ donor chimerism above the median (0.9%) on day +1–3 rejected, while 40% (6/15) of the patients with CD15+ donor chimerism below the median rejected (LogRank test, p=0.01). No differences were found in total number of nucleated cells, CD34+, CD3+, CD4+, or CD8+ cells in the donor harvest between patient who rejected and those who did not.
Conclusions: The kinetics of early CD15+ donor chimerism differed from the kinetics of early CD4+ and CD8+ donor chimerism. Early CD15+ donor chimerism was markedly lower than CD4+ and CD8+ donor chimerism. CD15+ donor chimerism increased toward 100% donor chimerism more rapidly than the CD4+ and CD8+ chimerism. Primary rejection of donor stem cells can be identified early, as these patients have no measurable donor CD4+ or CD8+ chimerism on day +1–3. Furthermore, patients with CD15+ donor chimerism above the median on day +1–3 have a low risk for rejection.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal