Abstract
Glucocorticoids are capable to modulate erythroid differentiation in vivo and in vitro. The reversible inhibition exerted by these factors on the maturation of CD36high CD235alow pro-erythroblasts was exploited to establish HEMA, a culture system that allows massive production (108−10 cells) of erythroblasts from as little as 10 mL of adult peripheral blood (
G. Migliaccio et al. Blood Cells Mol Dis 28:169, 2002
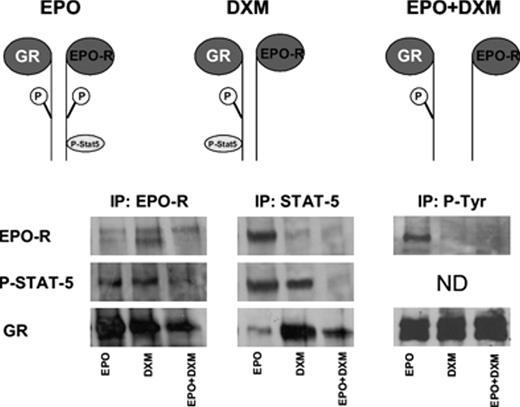
). How glucocorticoids block erythroid differentiation is not known. We hypothesized that this effect could be due to physical interaction of the glucocorticoid receptor (GR) either with the erythropoietin receptor (EPO-R), or with one of its downstream signalling partners. To test this hypothesis, CD36high CD235alow proerythroblasts were generated in HEMA culture, growth factor starved for up to 4 hrs and then stimulated either with EPO (3U/mL), Dexamethasone (DXM, 10−6M), or the combination of both, up to 4 hrs. Next, we undertook a series of immunoprecipitation (IP), followed by appropriate western blots (WB), of lysates from cells exposed to the different conditions. Both EPO and DXM rapidly (within 15 min) phosphorylated STAT-5, as shown by IP for STAT-5 followed by WB for P-STAT-5. In contrast, stimulation of the cells with both EPO and DXM for up to 4 hrs did not result in STAT-5 phosphorylation. On the other hand, IP with EPO-R was positive for EPO-R WB under all the conditions, but positive for P-STAT-5 WB and GR WB when cells were stimulated either with EPO or with DXM. The amount of GR detected by WB in EPO-R IP from cells stimulated with both EPO and DXM was greatly reduced (see Figure). In contrast, in STAT-5 IP, EPO-R was WB only in cells stimulated with EPO, P-STAT-5 was WB in cells treated with EPO or DXM, and GR was WB mainly in cells treated with DXM. In agreement with these results, IP for P-Tyr were positive for EPO-R only from cells treated with EPO, but were WB for GR with all the conditions. The fact that P-STAT-5 was WB in EPO-R IP from cells treated with EPO or with DXM, while EPO-R was WB in STAT-5 IP only from cells treated with EPO, is consistent with a model according to which, since EPO-R IP is positive for GR by WB, P-STAT-5 is IP with EPO as part of the EPO-R/GR/P-STAT-5 complex. These results suggest that EPO-R and GR are present in CD36high CD235alow cells stimulated with either EPO or DXM as a multicomplex, P-STAT-5 becames part of this complex in association with either EPO-R or GR, depending on the stimulus, EPO or DXM, to which the cells are exposed. Stimulation with both factors, leads to dissociation of the EPO-R/GR complex and neither of the two receptors is capable to stimulate phosphorylation of STAT-5. We propose that glucocorticoids interfere with erythroid differentiation by a non-genomic mechanism that involves physical interaction with EPO-R.Figure 1:
Summary of the IP with EPO-R, STAT-5 and P-Tyr specific antibody processed by WB for EPO-R, P-STAT-5 and GR, as indicated. A model summarizing the results is described on the top.
Figure 1:
Summary of the IP with EPO-R, STAT-5 and P-Tyr specific antibody processed by WB for EPO-R, P-STAT-5 and GR, as indicated. A model summarizing the results is described on the top.
Author notes
Corresponding author
2005, The American Society of Hematology
2005

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal