Abstract
Background. ATL is an HTLV-I-associated hematological malignancy. The majority of ATL patients are not curable using current chemotherapy with median survival time of 13 months. Recently, patients undergoing allo-SCT with conventional conditioning regimen were reported to have a 3-year overall survival (OS) rate of 45%, however, with high transplant-related mortality (TRM) rate as much as 40% (
Patients and Methods. Between Oct 2000 and Jun 2005, 16 ATL patients underwent RIST from a related donor. Since 3 have been enrolled into an on-going multi-center trial, 13 patients were analyzed in this retrospective study, including 3 who were enrolled into the above-mentioned multi-center trial (
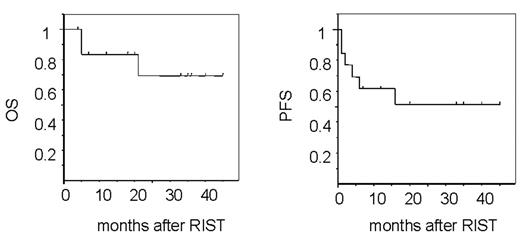
Results. Engraftment was rapid (median, 11 days) in all cases, and there was no TRM. Acute GVHD of grade II–IV developed in 7, and chronic extensive GVHD in 5. Eleven patients achieved CR after RIST, and 6 relapsed or progressed, among whom 2 achieved CR after cessation of CSP, donor lymphocyte infusion and local irradiation, and have been disease-free for more than 1 year. Ten patients are alive, 9 without disease, with median follow-up time of 26 mos (range, 4–45 mos). HTLV-I proviral titers determined using real time PCR decreased after RIST and became undetectable in 8 out of 13 patients. In 3, including one transplanted from an HTLV-I carrier donor, anti-HTLV-1 antibody transiently decreased and became nearly undetectable.
Conclusions. RIST is feasible and safe in ATL patients, and it has not only an anti-ATL but also an anti-HTLV-I activity. This is the first report documenting the efficacy of RIST for ATL in terms of survival and remission durability.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal