Abstract
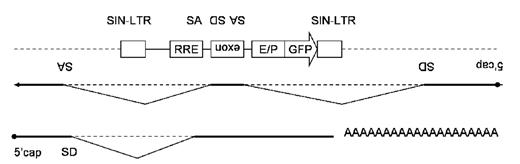
Retroviral transduction of hematopoietic stem cells is the powerful strategy to cure hereditable disease, especially severe congenital immunodeficiency. During past decade, most efforts were made to improve transduction efficiency and gene expression. Now the main concern shifted toward improvement of safety, in response to development of leukemia in SCID-X1 patients treated with retrovirus vector. Although recent large scale comparative studies suggest that lentiviral vector may be safer than oncoretroviral vector because of the difference of the integration pattern, insertional mutagenesis is still a major concern in these two vector systems. The most promising strategy to avoid insertional activation of proto-oncogene is the utilizing of an insulator element such as chicken beta-globin 5′HS4. However, no clear demonstration had been made regarding inhibition of insertional gene activation by the insulator element in the context of retroviral vectors. We have previously shown that the insertion of the1.2kb 5′HS4 insulator into LTR of SIN-HIV1 vector in forward or reverse orientation (INS1L and INS1R respectively) attenuated transducing ability by disturbing reverse-transcription, while insertion of the 0.25kb core insulator in forward or reverse orientation (C1R or C1L respectively) preserved transducing ability. And each insulator similarly protected gene expression from enhancer and silencer of near-by genome demonstrated by lower CV value of GFP positive cells distribution measured by FACS. In this experiment, we developed an in vivo exon trapping vector system to detect insertional gene activation. A short simian immunodeficiency virus derived sequence flanked by splice acceptor and donor sequence from human gamma-globin gene was inserted between RRE and the internal enhancer/promoter of the HIV-1 vector in reverse orientation. This artificial exon should enable in vivo bulk detection of inserted gene activation. The level of trapped gene expression measured by Q-RT-PCR of the parental vector, which harboring MSCV-U3 promoter as an internal promoter, was 2.0±0.1x104 copy per 100ng of total RNA after normalization of averaged vector copy number per diploid to 1 in 293T cells. To verify the trapping ability, point mutations were introduced into splice signals of artificial exon which reduced trapped signal and was 40% of the parental vector (7.6±0.6x103, p=0.0005). Imperfect reduction may be explained by the existence of splice acceptor site near RRE element, which still traps mRNA from reverse orientation. Averaged insertional gene activation level of parental vector was measured by in comparison with the vector which has no internal enhancer/promoter, and was about 1.6 fold (1.2±0.1x104, p<0.003). Finally, the level of enhancer blocking, from inside to outside, by the insulators were measured using this system. Unexpectedly, no reduction of near-by gene activation was observed with either insulator (INS1R, 2.3±0.1x104; INS1L, 1.9±0.3x104; C1R, 1.9±0.2x104; and C1L, 2.0±0.1x104). One hypothesis is that the insulators may activate inserted gene by modulating chromatin structure from barrier activity. This assay system is useful for in vivo trapping of gene activation but may need further analyses to study the risk of insertional mutagenesis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal