Abstract
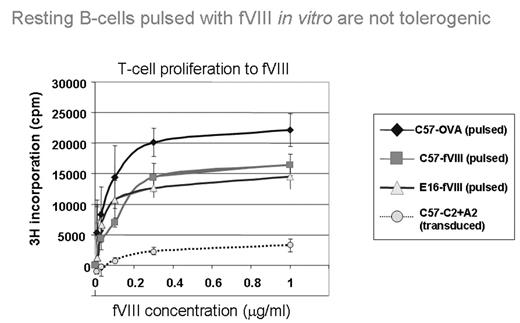
Tolerance induction for hemophilia inhibitor formation has recently been achieved by gene therapy using B cells transduced with a fusion protein of C2 and A2 domains of factor VIII with an IgG heavy chain. We have shown that inhibitor titers can be reduced over 90% by this approach in both naïve and highly immunized FVIII knockout mice (FVIII−/−). This process requires the presence of CD25+ regulatory cells for induction, and is stable for at least three months (Lei, T-C. and Scott, D.W. 2005. Induction of tolerance to fVIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B-cells as Ig fusion proteins. Blood, 105: 4865–4870). Herein, we wished to establish whether retroviral transduction and integration of the fusion Ig was necessary for tolerance. In addition, we also tested whether transduction of bone marrow could lead to long-term tolerance. B cells (resting and LPS blasts) from FVIII sufficient C57Bl/6 and FVIII−/− mice were pulsed with FVIII and injected into naïve FVIII−/− recipients that were subsequently challenged with therapeutic doses of FVIII in an immunogenic protocol. We reasoned that C57Bl/6 mice were FVIII sufficient and could display FVIII processed epitopes within their MHC class II. Moreover, pulsing with FVIII should lead to presentation of processed epitopes transiently in class II MHC. We found that specific tolerance to FVIII was only induced with B cells transduced with a combination of A2-IgG and C2-IgG, indicating that transient expression of FVIII epitopes and presentation by B cells is not sufficient for tolerance induction.
Resting B-cells pulsed with fVIII in vitro are not tolerogenic
In further studies, we examined whether tolerance could be induced by bone marrow transplantation of C2-Ig and A2-Ig transduced hematopoietic stem cells under a non-myeloablative regimen with busulfan in immunized FVIII−/− mice. This protocol could lead to long-term tolerance if pluripotential stem cells were transduced and engrafted. Our results indicate that tolerance to FVIII domains could be achieved even though the level of expression of GFP positive cells in peripheral blood of busulfan-treated mice was less than 1% (compared to >50% positive cells in lethally irradiated recipients of similarly transduced bone marrow cells). These results have implications for long-term therapy of inhibitor formation in hemophilia A. (Supported by NIH grants HL061883, AI035622 and a Lab Grant from the National Hemophilia Foundation.)
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal