Abstract
We have investigated the use of partially matched family member donors in combination with alemtuzumab and a non-myeloablatvie preparative regimen in an effort to expand the population of patients who may benefit from allogeneic immunotherapy.
Methods: Sixty three patients received fludarabine 30mg/sq m, cyclophosphamide 500mg/sq m IV qd x 4 days and alemtuzumab 20mg IV qd x 5 days followed by infusion of partially matched family member donor stem cells. Mycophenolate alone or in combination with cyclosporine was given for 6–8 weeks following transplantation.
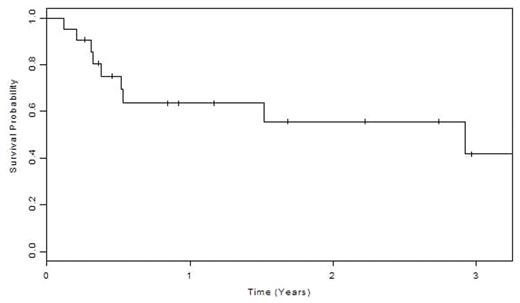
Results: Patient diagnoses included lymphoma/myeloma n= 16, leukemias/MDS n=33, myelofibrosis/aplasia n=3 and metastatic solid tumours n=11. The median age was 48 (range 17–66) with a median follow up of 28 months. The donor matching was 5/6 in 8, 4/6 in 22, and 3/6 in 33 cases. The median CD34+ cell dose collected was 13.47 x 106/kg (SD= 5.10) with nearly 2 logs of T cells depletion. Engraftment occurred in 92% of patients with a median of 87% donor cells responsible for patient hematopoiesis by 6 weeks following therapy. Twenty patients also received a DLI (range 105–107 CD3+ cells/kg). Grade III–IV acute GVHD occurred in only 8/63 (13%) with 14 (22%) experiencing grade II–IV. Nine patients developed chronic GVHD or failure to thrive (15%). The transplant regimen was well tolerated with 10% TRM. In those with hematologic malignancies, only 6 (12%) started in remission, though 38 (73%) attained a CR. The most common cause of death in this group was progressive disease (33%), followed by infections (19%), and GVHD (12%). Overall 1 year survival for this high risk group of 63 patients was 26%. A subgroup had aplastic anemia/myelofibrosis or leukemia/lymphoma in second or greater CR, PR and had a median disease free and overall survival of 3 years.
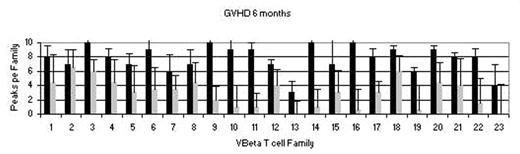
Phenotypic analysis of lymphocyte subsets (measured by flow), and T cell VBeta family recovery (spectratype analysis using PCR) revealed robust recovery by 6 months in those without GVHD (dark bars) compared to those with GVHD (gray bars), despite T depletion.
Further, the measured T cell recovery is from peripheral expansion of residual transplanted T cells as newly educated T cells are not measurable (using TRECs analysis) for at least 1 year, if at all.
Conclusions: The results demonstrate reasonable tolerance and reliable engraftment using T cell depleted, partially matched family member donors in a non-myeloablative setting with low treatment related mortality and severe GVHD. The future challenge will to be to develop strategies to improve immune recovery to enhance immune-mediated graft-versus-tumour effect and to minimize the risk of infections.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal